- Home
- »
- Clinical Diagnostics
- »
-
Liver Cancer Diagnostics Market Size & Share Report, 2030GVR Report cover
![Liver Cancer Diagnostics Market Size, Share & Trends Report]()
Liver Cancer Diagnostics Market Size, Share & Trends Analysis Report By Test Type (Laboratory Tests, Imaging, Endoscopy, Biopsy), By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-3-68038-012-5
- Number of Report Pages: 180
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Report Overview
The global liver cancer diagnostics market size was valued at USD 8,718.8 million in 2022 and is anticipated to expand at a compound annual growth rate (CAGR) of 6.67% by 2030. Around the world, liver cancer is among the leading cause of cancer-related deaths. As per the American Cancer Society, since the 1980s, liver cancer occurrence rates have increased three times and death rates have doubled now. According to cancer statistics in 2022, in the U.S., around 41,260 new cases are expected to be diagnosed whereas about 30,520 deaths will account because of liver cancer. The prevalence of liver cancer worldwide and the associated continuous exposure of the population to risk factors will drive the screening & diagnosis market rapidly.
The management of diseases and surveillance of health has significantly increased during the COVID-19 pandemic. This has increased the demand for rapid screening methods & point-of-care tests for the mitigation of outbreaks. However, during the pandemic, cancer care observed a disruption due to the allocation of funds & resources for COVID-19. For instance, as per an international survey, in March-June 2020, the liver cancer screening programs were canceled in over 80% of the centers, and treatments were delayed in most centers. But during the past year, with a deep understanding of COVID-19 and its pathophysiology, researchers have delved to assess the impact of COVID-19 on primary care for liver cancer services and encouraged patients to engage in routine healthcare screening. This will boost the early screening & diagnosis market for liver cancer as it will effectively control the mortality rates and result in better patient outcomes.
The risk factors for liver cancer include lifestyle changes such as smoking, alcohol consumption, and tobacco chewing, and diseases such as chronic viral hepatitis i.e. hepatitis B virus (HBV) or hepatitis C virus (HCV), cirrhosis, type 2 diabetes, and even obesity. The prevalence of one or more risk factors predisposes the liver and increases the probability of the occurrence of liver cancer. Accurate early detection, diagnosis & staging is important for improving survival rates. This has led to exhaustive initiatives undertaken by many institutes & companies to develop innovative solutions for screening. For example, in October 2022, researchers using the DELFI platform, an AI liquid biopsy technology by Delfi Diagnostics, Inc. presented the findings of accuracy in the detection of patients with liver cancer from patients without cancer, in a cost-effective manner.
Moreover, the growing number of government programs to spread awareness about early detection & diagnosis of liver cancer will also boost the worldwide demand for diagnostics solutions. For instance, in August 2022, the National Cancer Institute (NCI) sponsored researchers from Baylor College of Medicine, Texas with USD 5.5 million in a 5-year grant. The research will have a goal to reduce the burden of liver cancer by analyzing the risk factors, and identification of preventative treatments. Also, collaborative efforts and partnerships by the WHO, National Cervical Cancer Coalition, the U.S. Preventive Services Task Force, the CDC, and others for routine screening programs for liver cancers are key factors anticipated to fuel the growth during the forecast years.
The technologically advanced solutions for diagnosis are usually of high cost and are expected to restrain market growth. For developing countries like South Africa, Mexico, Colombia, and Iran, these expensive diagnostics are not affordable. This results in poor patient outcomes and increased susceptibility to liver cancers. Furthermore, with the constrained insurance & reimbursement frameworks in developing economies, access to screening tests is restricted.
Test Type Insights
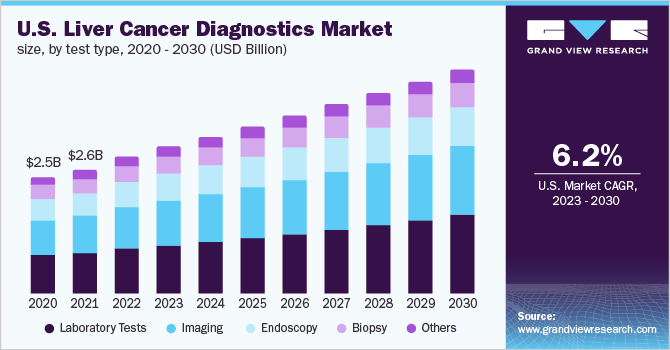
In terms of revenue, the laboratory tests segment dominated the market with a share of over 33.7% in 2022. This segment dominated due to key factors such as high preference by the population due to the accuracy & cost efficiency. The high-risk patients are screened primarily via laboratory tests and these tests help decide different lines of treatment. It also assists in further evaluation of the treatment plan and how it will affect other organs.
Furthermore, the laboratory tests segment is also projected to register the fastest CAGR during the forecast period. As laboratory tests are used for early screening, they can help medical professionals to identify the root cause of the tumor and determine the disease stage. These tests are often utilized to monitor the treatment efficacy, and evaluate if the tumor has come back post-treatment. Vast applications of laboratory tests for liver cancer also allow the segment to grow rapidly.
End-use Insights
In terms of revenue, the hospitals and diagnostic laboratories segment dominated the market with a share of over 49.57% in 2022. Hospitals are centers for primary diagnosis & care for a majority of the population. Conditions such as Hepatocellular Carcinoma (HCC), cholangiocarcinoma, and other conditions require facilities for disease management & treatment in a long-term manner. In addition, hospitals & diagnostic laboratories provide patients with cost-effective screening products. Moreover, the presence of skilled professionals, advanced techniques, sample collection & highly sensitive data in one place results in a short turnaround time. Thus, these settings are highly preferable and attribute to the highest revenue generation.
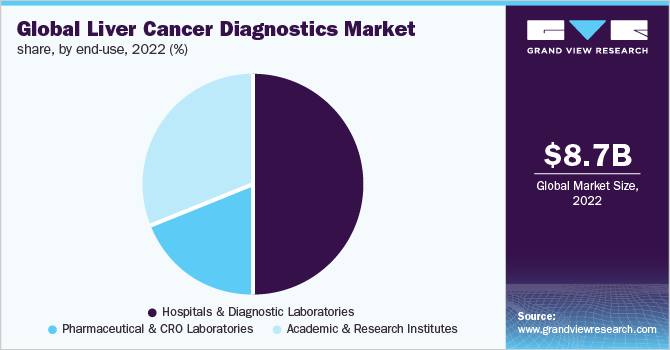
The pharmaceutical and CRO laboratories segment is expected to witness the fastest growth rate during 2023-2030. Owing to the mounting demand for diagnostic solutions, many CROs are providing services for liver cancer research, supporting the high growth. In addition, pharmaceutical companies are utilizing advanced research and developing new products in the market. For example, in October 2022, BioCartis Group N.V. announced the commercialization of its test kit, HepatoPredict, in Europe. The CE-IVD test was developed by its partner, Ophiomics, and is a prognosis kit for liver transplant in HCC patients.
Regional Insights
North America dominated the liver cancer diagnostics market with a share of 40.37% in 2022 and is projected to generate the highest revenue during the forecast period. The well-established healthcare settings, extensive reimbursement framework, rising implementation of novel diagnostics, and adoption of healthcare insurance policies among the population contribute to the dominance of this region in the market. Additionally, due to the increasing incidence of various tumor cases, supportive government organizations like CDC, NIH, and IARC, among others in this region, also boost the research & development of new testing & screening methods. Furthermore, the presence of market players in North America allows for easy and early access to novel solutions worldwide.
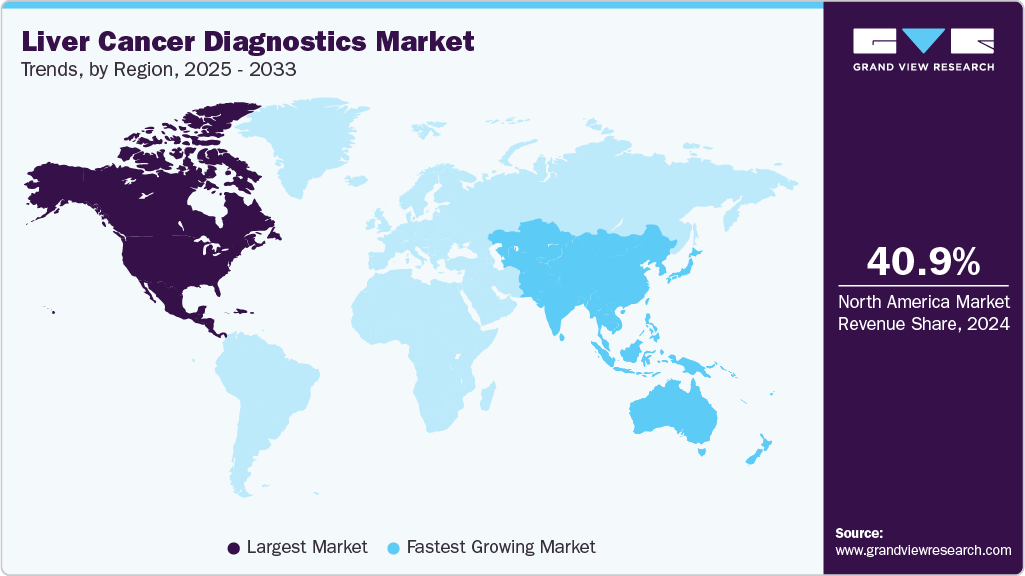
Asia Pacific is anticipated to observe the highest growth during the forecast years. The high growth to be registered can be attributed to the significant growth of the geriatric population exposed to risk factors in this region. Additionally, other factors such as increasing initiatives by governments & private organizations to improve access to healthcare are driving the early detection market. For example, in August 2021, Berry Oncology secured funds of USD 99.1 million for the large-scale cohort study for liver cancer screening in China. The cohort study uses the NGS platform to analyze the liquid biopsy samples.
Key Companies & Market Share Insights
The liver cancer diagnostics market space is growing rapidly and by the adoption of strategic initiatives, key players are also expanding their presence. For example, in January 2022, Thermo Fisher Scientific, Inc. collaborated with OncoCyte Corporation, a California-based diagnostics company. With this agreement, the company will expand its offering of in-vitro diagnostics assays of precision oncology. Developments like these will propel market growth and result in improved patient outcomes. Some of the prominent players in the liver cancer diagnostics market include:
-
Abbott Laboratories
-
Thermo Fisher Scientific, Inc.
-
F. Hoffmann-La Roche Ltd.
-
Qiagen N.V.
-
Siemens Healthineers
-
Becton, Dickinson & Company
-
Illumina, Inc.
-
Epigenomics AG
-
Koninklijke Philips N.V.
-
Fujifilm Medical Systems U.S.A., Inc.
Liver Cancer Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 9,339.6 million
Revenue forecast in 2030
USD 14.67 billion
Growth rate
CAGR of 6.67% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD Million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Test type, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Rest of North America; U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Rest of Europe; Japan; China; India; South Korea; Australia; Thailand; Rest of Asia Pacific; Brazil; Mexico; Argentina; Rest of Latin America; South Africa; Rest of Middle East & Africa; UAE; Kuwait; Saudi Arabia
Key companies profiled
Abbott Laboratories; F. Hoffmann-La Roche Ltd.; Qiagen N.V.; Thermo Fisher Scientific, Inc.; Siemens Healthineers; Becton Dickinson & Company; Illumina, Inc.; Koninklijke Philips N.V; Epigenomics AG; Fujifilm Medical Systems U.S.A., Inc.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Liver Cancer Diagnostics Market Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global liver cancer diagnostics market report on the basis of test type, end-use, and region:
-
Test Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Laboratory Tests
-
Biomarkers
-
Oncofetal and Glycoprotein Antigens
-
Enzymes and Isoenzymes
-
Growth Factors and Receptors
-
Molecular Markers
-
Pathological Biomarkers
-
-
Blood Tests
-
-
Imaging
-
Endoscopy
-
Biopsy
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals & Diagnostic Laboratories
-
Academic & Research Institutes
-
Pharmaceutical & CRO Laboratories
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Rest of North America
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
Rest of Europe
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
Rest of Asia Pacific
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
Rest of Latin America
-
-
Middle East and Africa (MEA)
-
South Africa
-
UAE
-
Kuwait
-
Saudi Arabia
-
Rest of Middle East & Africa
-
-
Frequently Asked Questions About This Report
b. Based on test type, laboratory tests accounted for the largest share of 33.75% in 2022. The high market share can be attributed to increased demand for the effective diagnosis of hepatocellular carcinoma.
b. Some key players operating in the liver cancer diagnostics market include Abbott Laboratories; F. Hoffmann-La Roche Ltd.; Qiagen N.V.; Thermo Fisher Scientific, Inc.; Siemens Healthineers; Becton Dickinson & Company; Illumina, Inc.; Koninklijke Philips N.V.; Epigenomics AG; and Fujifilm Medical Systems U.S.A., Inc.
b. The global liver cancer diagnostics market size was estimated at USD 8,718.8 million in 2022 and is expected to reach USD 9,339.6 million in 2023.
b. The global liver cancer diagnostics market is expected to witness a compound annual growth rate of 6.67% from 2023 to 2030 to reach USD 14.67 billion in 2030.
b. The major factors driving the liver cancer diagnostics market growth are the introduction of innovative diagnostic products and the rising prevalence of liver cancer.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





