- Home
- »
- Medical Devices
- »
-
Medical Device Complaint Management Market Report, 2030GVR Report cover
![Medical Device Complaint Management Market Size, Share & Trends Report]()
Medical Device Complaint Management Market (2025 - 2030) Size, Share & Trends Analysis Report By Service Type (Complaints Log/Intake, Product Surveillance & Regulatory Compliance, Returned/ Non-returned Product Analysis), By Region, And Segment Forecasts
- Report ID: GVR-2-68038-783-4
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
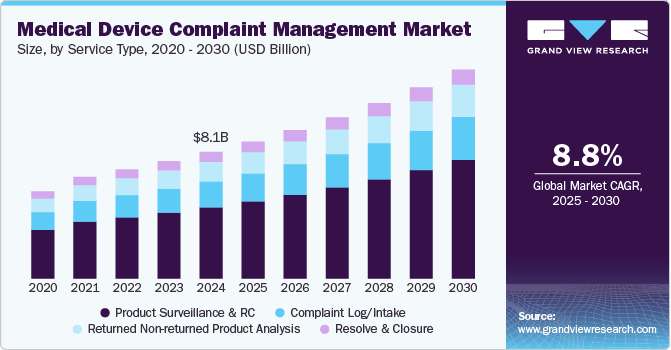
The global medical device complaint management market size was estimated at USD 8.08 billion in 2024 and is expected to grow at a CAGR of 8.79% from 2025 to 2030. The growing adoption of digitalization in all phases and functionalities of the healthcare industry is promoting market growth. Rapidly transforming systems from paper-based to digital solutions in managing complaints are supporting market growth. The rising number of public and private partnerships and favorable government initiatives are boosting the adoption rates of automated digital systems in complaint management processes.
The implementation of these automated software supports enhanced documentation. By using a multi-page electronic form, complaint data and information are accurately and precisely captured and are reorganized by the software solution into a three-step program that comprises complaint processing, investigation, and resolution. Every step of this program is computerized and precisely documented. Furthermore, the complaint management system offers advanced analytical solutions incorporated with reporting tools, which are used in augmented management oversight, providing required transparency that enhances the decision-making processes.
Moreover, key participants are constantly focusing on devising innovative product development strategies to gain a competitive edge and cater to the growing demand in the market. Companies are developing innovative mobile application solutions to enhance customer complaints management. For instance, Intellect offers an integrated mobile application platform allowing customers to register complaints, escalate ticks, and generate queries. The Intellect mobile application is easily available on the App Store and the Google Play Store and can be downloaded as an Intellect application or a custom-branded mobile application.
Favorable government initiatives are boosting the growth of the complaint management market. For instance, the U.S. FDA encourages patients, healthcare professionals, care providers, and consumers to voluntarily submit reports of product defects and/or adverse events to MedWatch, which is the U.S. FDA’s Safety Information and Adverse Event Reporting Program, or then by using MedWatcher, the mobile application. Moreover, adverse event reporting witnessed a surge in the number owing to the launch of the MedWatcher. The MedWatcher application simplifies and accelerates the complaint reporting process by offering customers an easy four-step form, which is to be electronically submitted to the U.S. FDA. Therefore, the development and launch of these innovative mobile applications in complaint management are expected to fuel market growth.
The growing emphasis on patient-centric solutions provides organizations with opportunities to improve user experience and satisfaction, which in turn leads to more effective processes for resolving complaints. Companies that can optimize their complaint management systems and respond swiftly to customer feedback are likely to achieve a competitive advantage. Recently, there has been an increasing trend towards digital transformation in the medical device sector. Numerous organizations are implementing cloud-based complaint management systems that facilitate real-time tracking and analysis of complaints. This transition not only boosts operational efficiency but also fosters better collaboration among teams, resulting in faster decision-making.
Market Characteristics & Concentration
The chart below represents the relationship between industry concentration, industry characteristics, and industry participants. The x-axis shows the level of industry concentration, ranging from low to high. The y-axis represents various market characteristics, such as degree of innovation, impact of regulations, industry competition, service and expansion, level of partnerships and collaboration activities, and regional expansion. For instance, the industry is fragmented, with services and end users entering the market. There is a moderate degree of innovation, a medium level of partnerships and collaboration activities, a moderate impact of regulations, and a moderate geographical expansion of the industry.
The industry is experiencing a moderate degree of innovation. As the focus on delivering better and more efficient care increases, the demand for medical device complaint management platforms is expected to grow significantly, contributing to the overall advancement of the healthcare industry. The use of cloud-based platforms for real-time data sharing among stakeholders such as manufacturers, healthcare providers, and regulatory bodies. This facilitates faster communication and collaboration when addressing complaints.

The regulatory framework involves compliance with industry-specific guidelines and standards to ensure accuracy and consistency. For instance, regulatory agencies are increasingly emphasizing post-market surveillance through innovative methodologies such as real-world evidence (RWE). By utilizing data from actual clinical settings rather than controlled trials alone, companies can gain insights into how devices perform over time in diverse populations. This approach helps in identifying long-term safety concerns that may not have been evident during the initial testing phases.
Geographical expansion helps companies increase their presence to maintain or strengthen their market presence. For instance, Medtronic, which operates in over 150 countries, has established regional complaint-handling systems that are tailored to local regulations and languages. This allows them to respond quickly to complaints while ensuring compliance with local laws. Additionally, they utilize centralized databases that aggregate complaint data from various regions, enabling them to identify trends and address systemic issues more efficiently.
Service Type Insights
Based on service type, the product surveillance and regulatory compliance segment dominated the market, with the largest revenue share of over 56% in 2024. The growth is driven by regulatory agencies, which are important for making sure medical devices are safe and work well. There is more focus on monitoring these products because people are more aware of the risks that come with medical devices. Problems such as injuries, defects, and device failures have serious consequences, including harm or even death. Because of this, both regulatory agencies and consumers are less willing to accept these issues.
In addition, regulatory changes have been made by organizations like the U.S. FDA. For instance, the U.S. FDA requires companies to report any complaints about problems or failures with medical devices directly to them. If companies do not follow these rules, they could face heavy penalties.
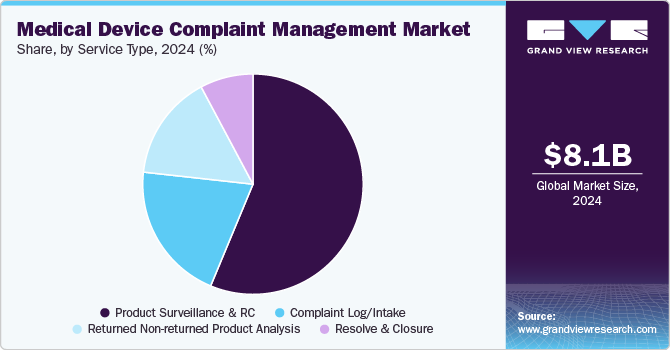
The complaints log/intake segment is expected to grow at the fastest CAGR of 8.96% over the forecast period. This segment is the pioneer stage of complaint management and portrays a gateway mechanism for preventive or corrective action and post-market activities. Complaint files are linked to the medical device reporting (MDR) event file since the complaint needs to be assessed and determined whether it is a reportable adverse event. Moreover, it is compulsory for medical device manufacturers to capture and track complaints based on the quality system regulation (QSR), which has been defined in 21 CFR Part 820.
Regional Insights
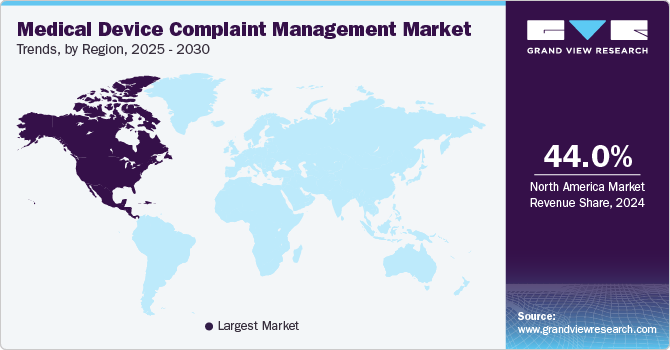
North America medical device complaint management market dominated the global industry with the largest revenue share of over 44% in 2024. This growth is attributed to the increasing emphasis on post-market surveillance as manufacturers recognize the importance of monitoring device performance aftermarket release. This trend is evident in the growing adoption of software solutions that facilitate real-time monitoring of device performance and user feedback. Companies are investing in comprehensive complaint management systems that not only capture complaints but also provide insights into product improvements based on user experiences.
U.S. Medical Device Complaint Management Market Trends
The medical device complaint management market in U.S. held the largest share in 2024 due to the presence of stringent regulatory guidelines for complaint management. The U.S. Food and Drug Administration (FDA) has implemented stricter guidelines for reporting adverse events related to medical devices. For instance, FDA’s focus on post-market surveillance has led manufacturers to enhance their reporting mechanisms to ensure compliance with regulations such as 21 CFR Part 803, which mandates timely reporting of adverse events related to medical devices. This regulatory pressure compels companies to invest in sophisticated software solutions that streamline complaint handling processes.
Europe Medical Device Complaint Management Market Trends
The medical device complaint management market in Europe is anticipated to grow significantly due to the presence of developed economies, such as Germany, the UK, France, Spain, & Italy. Moreover, manufacturers are increasingly recognizing the importance of direct communication with patients regarding device-related issues. This trend is exemplified by Philips Healthcare’s initiatives to create platforms where patients can report issues directly. One of their key initiatives is the development of platforms that allow patients to report issues directly related to their healthcare experiences. This approach not only empowers patients but also fosters a culture of transparency and trust between healthcare providers and patients, thus fostering transparency and trust.
The UK medical device complaint management market is expected to grow significantly over the forecast period. In May 2024, the Medicines and Healthcare Products Regulatory Agency (MHRA) released a statement outlining its policy intent regarding the international recognition of medical devices. This document details how the UK Government plans to acknowledge regulatory approvals from several countries, including Australia, Canada, the European Union, and the United States. The recognition will vary based on factors such as device type, classification, and prior approval status. The MHRA is actively reviewing its list of countries with comparable regulatory frameworks and is currently engaged in discussions with Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) to consider recognizing medical device approvals from Japan as well. The primary aim of this policy is to ensure that patients have safe access to high-quality medical devices while minimizing redundant assessments by regulators that are deemed comparable
The medical device complaint management market in Germany held a significantshare in 2024 due to the increasing complexity of medical devices, regulatory requirements, and the rising emphasis on patient safety. Companies are actively investing in advanced complaint management systems to streamline processes and ensure compliance with the stringent regulations set forth by authorities such as the Federal Institute for Drugs and Medical Devices (BfArM) and the European Medicines Agency (EMA). These initiatives include implementing robust software solutions that facilitate real-time tracking of complaints, enhancing data analytics capabilities to identify trends and root causes of issues, and fostering a culture of transparency within organizations.
Asia Pacific Medical Device Complaint Management Market Trends
The medical device complaint management market in Asia Pacific is anticipated to grow lucratively over the forthcoming years. The presence of renowned multinational companies such as Wipro and Tata Consultancy Services (TCS) spearheading the complaint management market in the Asia Pacific is expected to drive the market in Asia Pacific. Moreover, in February 2024, the Therapeutic Goods Administration (TGA) in Australia raised concerns regarding the management of medicine shortages and the discontinuation of certain products, which significantly influences the medical device complaint management market in the Asia-Pacific region. As healthcare providers and manufacturers face increased scrutiny over their supply chains and product availability, there is a heightened need for robust complaint management systems to address potential issues arising from these shortages. TGA’s inquiries compel medical device manufacturers to enhance their reporting mechanisms and ensure compliance with regulatory standards, thereby driving investments in complaint management solutions.
Japan medical device complaint management market held a significant revenue share in 2024, driven by an increasing demand for advanced healthcare solutions and a robust regulatory framework. Companies are focusing on enhancing their complaint management systems to ensure compliance with stringent regulations set forth by the Pharmaceuticals and Medical Devices Agency (PMDA) and the Ministry of Health, Labour, and Welfare (MHLW). This includes implementing comprehensive quality management systems that align with international standards such as ISO 13485. To facilitate this growth, companies are investing in digital technologies, such as artificial intelligence and data analytics, to streamline complaint handling processes. These technologies enable real-time monitoring of product performance and customer feedback, allowing for quicker resolution of issues.
The medical device complaint management industry in India is driven by the presence of stringent regulatory guidelines for complaint management. For instance, in September 2024, the Indian government introduced the Uniform Code for Marketing Practices in Medical Devices (UCMPMD) 2024, which aims to regulate and improve ethical practices within the medical device industry. A significant aspect of this code is the establishment of Ethics Committees for Marketing Practices in Medical Devices (ECMPMD) by all medical device associations. These committees are tasked with handling complaints related to any breaches of the UCMPMD. Upon receiving a complaint regarding a breach of the code, the ECMPMD is required to conduct an inquiry and reach a decision within 90 days. This timeline is crucial as it ensures that complaints are addressed promptly, promoting accountability and transparency within the industry.
Latin America Medical Device Complaint Management Market Trends
The medical device complaint management market in Latin America is driven by an increasing demand for advanced healthcare solutions and a rising awareness of patient safety. Companies are actively investing in complaint management systems to enhance their operational efficiency and compliance with regulatory standards. This growth is largely attributed to the expansion of healthcare infrastructure, increased access to medical technology, and a growing population that demands higher-quality healthcare services.
Brazil medical device complaint management industry held the largest market share in 2024 due to the increasing demand for healthcare services, advancements in medical technology, and a heightened focus on patient safety. Regulatory bodies such as ANVISA (Agência Nacional de Vigilância Sanitária) have implemented stringent guidelines that necessitate robust complaint handling processes. This system is crucial for ensuring the safety and efficacy of medical devices after they have been placed on the market. The primary regulations governing this system are outlined in Resolution RDC No. 67/2009 and Resolution RDC No. 551/2021. In response, companies are investing in advanced software solutions that facilitate real-time tracking of complaints, data analytics for identifying trends, and automated reporting systems to streamline communication with regulatory authorities.
Middle East And Africa Medical Device Complaint Management Market Trends
The medical device complaint management market in the Middle East & Africa is anticipated to grow significantly during the forecast period due to an increase in local manufacturing capabilities, which necessitate robust complaint management processes to maintain product quality and safety standards. Companies are investing in research and development to innovate new products while ensuring that existing devices meet stringent safety requirements. Overall, these initiatives reflect a commitment to enhancing patient outcomes through improved medical device oversight.
South Africa medical device complaint management industry is expected to grow significantly over the forecast period. The growth is driven by firms leveraging technology such as data analytics and AI to predict potential issues before they escalate into significant problems. By analyzing trends from complaint data, companies can identify recurring issues with specific devices or manufacturers, allowing them to take proactive measures. Moreover, there is a growing emphasis on transparency; companies are increasingly sharing information about their complaint management processes with stakeholders, which builds trust and enhances their reputation in the market.
Key Medical Device Complaint Management Company Insights
The industry is fragmented, with the presence of many country-level smart healthcare providers.The market players undertake several strategic initiatives, such as partnerships & collaborations, service launches, mergers & acquisitions, and geographical expansion to maintain their position and grow in the market.
Key Medical Device Complaint Management Companies:
The following are the leading companies in the medical device complaint management market. These companies collectively hold the largest market share and dictate industry trends.
- IQVIA
- Wipro
- Tata Consultancy Services (TCS)
- MasterControl
- Biovia
- Freyr
- Sparta Systems
- SAS
- AssurX
- Parexel International Corporation
Recent Developments
-
In June 2022, MasterControl formed a partnership with Elemental Machines to transform biomanufacturing by integrating digital production with the Internet of Things (IoT). This collaboration aims to enhance efficiency and innovation in the biomanufacturing sector, which could indirectly impact medical device complaint management.
-
In October 2021, the Oracle Health Sciences Complaints Management Cloud Service was launched to assist manufacturers in managing product complaints effectively while ensuring compliance with regulatory requirements. This service is particularly relevant for organizations in the healthcare and life sciences sectors, where managing complaints is critical for maintaining product quality and safety.
Medical Device Complaint Management Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 8.74 billion
Revenue forecast in 2030
USD 13.33 billion
Growth rate
CAGR of 8.79% from 2025 to 2030
Actual data
2018 - 2024
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million/billion, and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Service type, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Mexico, Germany, U.K., France, Italy, Spain, Norway, Denmark, Sweden, China, Japan, India, South Korea, Australia, Thailand, Brazil, Argentina, Saudi Arabia, South Africa, UAE, Kuwait
Key companies profiled
Tata Consulting Services (TCS); IQVIA; Biovia; Wipro; Sparta Systems; AssurX; Freyr; SAS; Parexel International Corporation; MasterControl
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Medical Device Complaint Management Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global medical device complaint management market report based on service type, and region:
-
Service Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Complaints Log / Intake
-
Receive Complaints
-
Classify the Issue
-
Record Issue
-
-
Product Surveillance & Regulatory Compliance
-
Reportable/Non-Reportable
-
Medical Device Vigilance/Medical Device Reporting
-
Field Action
-
-
Returned/ Non-Returned Product Analysis
-
Complaint Investigation
-
Root Cause Analysis, Testing
-
Corrective/Preventive Action
-
-
Resolve & Closure
-
Complaint Summary
-
Customer Letter Creation
-
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
U.K
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global medical device complaint management market size was estimated at USD 8.08 billion in 2024 and is expected to reach USD 8.74 billion in 2025.
b. The global medical device complaint management market is expected to grow at a compound annual growth rate of 8.79% from 2025 to 2030 to reach USD 13.33 billion by 2030.
b. Product surveillance and regulatory compliance services dominated the medical device complaint management market with a share of over 56% in 2024. This is attributable to the fact that the regulatory agencies and users of medical devices are becoming less tolerant of the failure of medical devices as it results in serious injury, death, or potential harm to the users.
b. Some key players operating in the medical device complaint management market include Tata Consulting Services (TCS); IQVIA; Biovia; Wipro; Sparta Systems; AssurX; Freyr; SAS; Parexel International Corporation; MasterControl.
b. Key factors driving the medical device complaint management market growth include the transition from paper-based to digital systems for tracking complaints, supportive government reforms, and increasing demand for an automated system for complaint management.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










