- Home
- »
- Medical Devices
- »
-
Medical Device Testing Services Market Size Report, 2030GVR Report cover
![Medical Device Testing Services Market Size, Share & Trends Report]()
Medical Device Testing Services Market Size, Share & Trends Analysis Report By Service (Biocompatibility Tests, Chemistry Test, Microbiology & Sterility Testing, Package Validation), By Phase, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-2-68038-115-3
- Number of Report Pages: 137
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Market Size & Trends
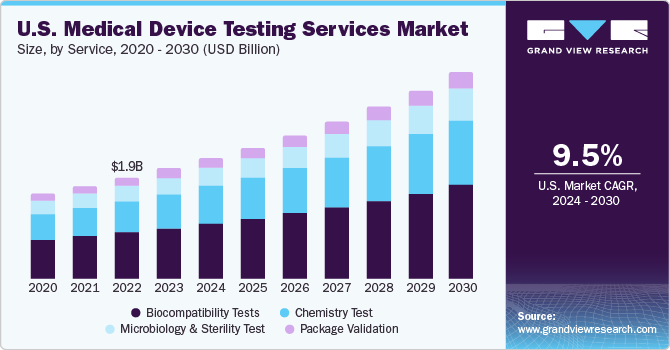
The global medical device testing services market size was valued at USD 8.95 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 9.44% from 2024 to 2030. Complexity in product design, intensifying competition, increasing number of small-sized medical device manufacturers, and strict approval norms are key factors driving the market. In addition, the COVID-19 pandemic has accelerated the demand for testing services around the globe. Favorable government support is expected to drive the market. To obtain approvals & clearances, companies need to comply with an elaborate list of standards provided by regulatory bodies and maintain complete documentation of the same.
These requirements must be met as per the stipulated formats and specifications to ensure the products are permitted for sale. The regulatory authorities also perform routine post-market surveillance by levying a fee on the manufacturer. Receipt of any complaints about drawbacks of the product can entail its withdrawal from the market, thereby proving the stringent nature of the regulations governing these procedures.
Market Concentration & Characteristics
Market growth stage is stable, and market growth is expected to accelerate over the estimated time period. The Medical Device Testing Services market is characterized by regulatory considerations, evolving technologies, materials innovation, and globalization & outsourcing of services.
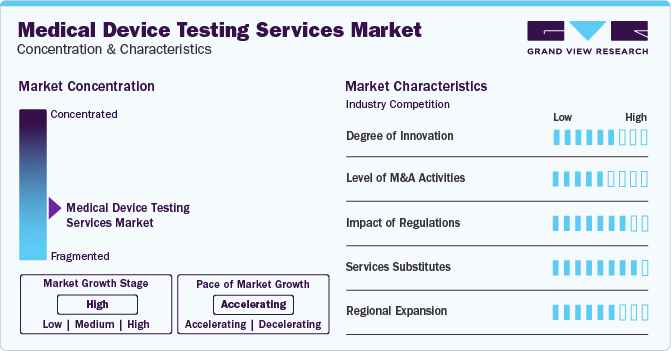
Furthermore, outsourcing in the medical device testing services market is substantially growing due to various benefits such as cost-effectiveness, access to specialized expertise, and flexibility in resource allocation. Likewise, outsourcing mitigates risks associated with in-house testing, offering flexibility in resource allocation, scalability, and access to a broader range of testing capabilities based on project requirements, thereby influencing market demand.
In addition, the market is highly competitive, characterized by numerous established and emerging players offering a wide array of testing services. Differentiation based on expertise, technology, regulatory compliance, and service quality is crucial for market positioning. Companies leveraging advanced types of medical device testing technologies and innovative methodologies gain a competitive edge. Investment in cutting-edge equipment and the ability to offer specialized, high-value testing solutions strengthens market growth.
ASCA's emphasis on conformity assessment encourages advancements in testing methodologies and technology adoption. Accredited labs are encouraged to continually improve and innovate, aligning with evolving regulatory expectations. Besides, innovations in testing technologies and methodologies, such as the adoption of AI, IoT, or robotics in testing processes, can significantly influence the medical device testing services market. Businesses need to stay abreast of technological advancements to remain competitive and meet clients' evolving needs.
Regulatory bodies, such as the U.S. FDA and EMA, continually upgrade the regulatory guidelines for safety, efficacy, & quality standards for medical devices. This escalation in regulatory requirements compels medical device manufacturers to conduct more comprehensive and rigorous testing to meet these stringent standards. Stringent approval norms necessitate specialized and extensive testing protocols. Medical device testing service providers offer expertise in conducting various specialized tests, including biocompatibility assessments, chemistry testing, sterility testing, and usability studies aligning with the heightened regulatory requirements, propelling market progression.
Actions and strategies of competitors within the medical device testing services market are crucial influencers. Understanding the competitive landscape helps businesses position themselves effectively and differentiate their offerings. Besides, several key players are acquiring smaller ones to strengthen their market position. Sharing expertise and resources facilitates the development of advanced testing methodologies and accelerates technological advancements in the industry and drives market growth. M&A activities efforts enable companies to increase their capabilities, expand their service portfolios, and improve their competencies, where testing services are tailored to meet specific device needs. This approach emphasizes fostering stronger relationships & delivering more targeted testing solutions.
Medical device companies across the globe are facing a diverse array of socioeconomic challenges. In developed economies, such as the U.S., there is pricing pressure. Hence, operators are exploring ways to reduce costs throughout the value chain. On the other hand, developing economies are showing major growth potential, despite a higher likelihood of being price sensitive. Hence, market players today are striving to reduce the overall cost of devices. Outsourcing testing operations helps companies focus on product development and enhance marketing efforts.Thus, numerous providers are entering in the market to offer robust testing services ensuring quality assurance further mitigating risks associated with product failures, enhancing overall market competitiveness
Changes in global healthcare trends, emerging markets, and shifts in research priorities can impact the demand for medical device testing services. Analyzing market influencers involves understanding the broader industry trends that may shape the market. Besides, the growing presence of local market players along with competitive pricing, rapid technological advancements, growing demand for new & cost-effective medical devices, and high healthcare expenditure fuels the market growth. Other factors contributing to market growth are rising investments, growing medical devices industry, and high standards for medical device development in the country.
Service Insights
On the basis of service segment, the market is classified into biocompatibility testing, chemistry testing, microbiology & sterility testing, and package validation. Biocompatibility tests held the largest share in 2023. Increasing demand for different types of medical device testing across various healthcare sectors, including cardiovascular, orthopedic, diagnostic devices, and several others, propels the market growth. Furthermore, stringent regulations, technological advancements, and a focus on personalized healthcare are expected to boost the growth of biocompatibility testing within the medical device testing services market.
Besides, the chemistry test segment is expected to grow at a lucrative CAGR of 9.72%. Medical devices are subjected to chemistry tests with pharmaceutical formulations in the medical industry. The chemistry tests support the medical device industry throughout the value chain. These tests ensure that medical devices do not cause reactions when in contact with the human body.
Phase Insights
On the basis of phase segment, the market is segregated into preclinical and clinical. Furthermore, the preclinical segment is subdivided into small animals, large animals, and others. The clinical segment held the largest market share in 2023. The segment growth is due to a rise in product pipeline and continuous upgradation of regulatory standards. Growing emphasis on clinical evidence for new device approvals accelerates the demand for robust testing services during clinical trials. Moreover, the growing preference for market participants that offer advanced testing methodologies with affordable cost & reduced turnaround time for diverse clinical studies is expected to drive segment growth.
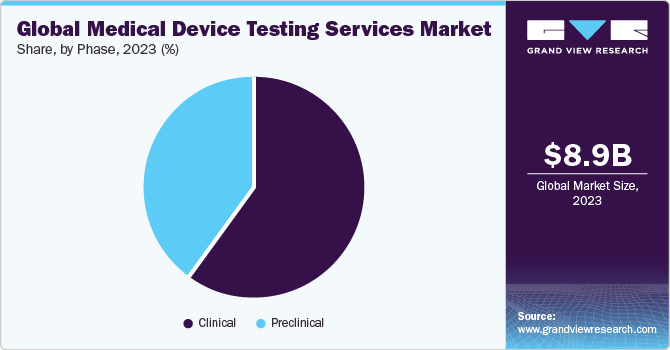
The preclinical market accounted for the 39.74% share in 2023. Preclinical testing is crucial in the development & evaluation of medical devices before they are approved for human use. The tests are used to identify the efficacy, safety, and performance of the devices, to mitigate potential risks & enhance patient outcomes. Furthermore, in preclinical among small animals, large animals, and others. The demand for small animal research is increasing due to the rise of domestic medical device manufacturers putting more effort into developing medical devices that can be used for such research. Besides, it helps address regulatory safety concerns and reduce risks associated with product validation. Likewise,large animal research is driven by an increase in the demand for medical device trials in order to bridge translation to human trials. Therefore, such factors are anticipated to contribute to market growth.
Regional Insights
Asia Pacific dominated the global medical device testing services market in 2023, holding a revenue share of 41.1%. Rapid advancements in healthcare infrastructure, coupled with increasing regulatory management, are driving the region's prominence in the global medical device testing services market. Moreover, the growing demand for various medical device testing methods, favorable pricing model and the rise of local manufacturing hubs propel the need for high-quality testing services, fostering innovation & technological expertise within the region. Moreover, cost-effective testing services and the availability of a skilled workforce are key factors driving regional revenue growth.
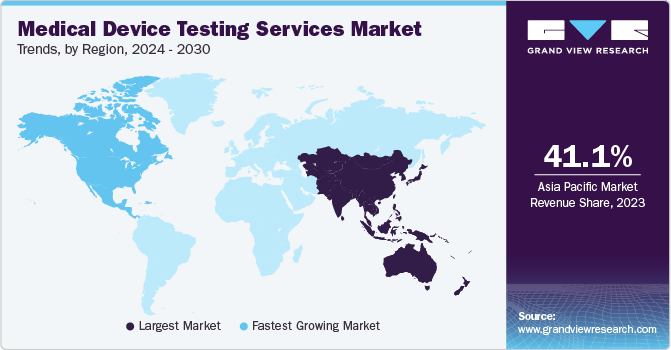
North America is estimated to show growth at a lucrative rate in the market over the forecast period. This can be attributed to a continuous owing to the presence of established manufacturing hubs of highly reliable, complex, and high-end medical devices in the region. In addition, many Original Equipment Manufacturers (OEMs) have shifted their focus to electronics manufacturing service providers to efficiently handle the increasing volume of electronic components in current medical devices. This has led to the rise in demand for medical device testing activities for efficient healthcare in the region and is expected to be one of the major factors propelling the development of the market.
Indian Medical Device Clinical Trial Market
In India, the medical device clinical trial is driven by increase in government funding for R&D to accelerate new product development which has the country as favored destinations for manufacturing services. Moreover, the country presents a market opportunity for international medical device manufacturers & is keen to expand its potential as a domestic manufacturing base & climb up the value chain. Therefore, outsourcing medical device testing services to emerging countries, and rising number of clinical trials is anticipated to fuel the global medical device testing services market over the forecast period.
Key Companies & Market Share Insights
Some of the medical device testing companies operating in the market are SGS SA, Laboratory Corporation of America Holdings, Nelson Laboratories, LLC, TÜV SÜD, Charles River Laboratories, Element Minnetonka, North America Science Associates Inc. (NAMSA), Eurofins Scientific, Pace Analytical Services LLC, Intertek Group Plc, and WuXi AppTec among others. Several key players are acquiring various strategic initiatives to strengthen their market position offering diverse services to customers. The prominent strategies adopted by companies are service launches, mergers & acquisitions/joint ventures merger, partnership & agreements, expansions, and others to increase market presence & revenue and gain a competitive edge. These initiatives may be implemented to expand regionally or widen the global network to reach more customers.
Key Medical Device Testing Services Companies:
The following are the leading companies in the medical device testing services market. These companies collectively hold the largest market share and dictate industry trends. Financials, strategy maps & products of these medical device testing services companies are analyzed to map the supply network.
- SGS SA
- Laboratory Corporation of America Holdings
- Nelson Laboratories, LLC
- TÜV SÜD
- Charles River Laboratories
- Element Minnetonka
- North America Science Associates Inc. (NAMSA)
- Eurofins Scientific
- Pace Analytical Services LLC
- Intertek Group Plc
- WuXi AppTec
Recent Developments
-
In November 2023, Intertek announced its partnership with Emitech Group, a French independent testing & engineering specialist.
-
In June 2023, TÜV SÜD opened a new laboratory in Minnesota. It is ISO 17025 accredited for the biological & chemical testing of medical devices. It is a part of the company’s commitment to providing high-quality medical device services.
-
In April 2023, Nelson Labs announced that the company is ASCA-accredited testing laboratory by the U.S. FDA within the category of biocompatibility.
Medical Device Testing Services Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 9.76 billion
Revenue forecast in 2030
USD 16.78 billion
Growth Rate
CAGR of 9.44% from 2024 to 2030
Historical Year
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Service, phase, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, Netherlands, Belgium, Switzerland, Japan, China, India, Australia, South Korea, Malaysia, Indonesia, Singapore, Philippines, Thailand, Brazil, Mexico, Argentina, Colombia, Chile, South Africa, Saudi Arabia, UAE, Kuwait, Israel
Key companies profiled
SGS SA, Laboratory Corporation of America Holdings, Nelson Laboratories, LLC, TÜV SÜD, Charles River Laboratories, Element Minnetonka, North America Science Associates Inc. (NAMSA), Eurofins Scientific, Pace Analytical Services LLC, Intertek Group Plc, WuXi AppTec
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Medical Device Testing Services Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global medical device testing services market report based on service, phase and region.
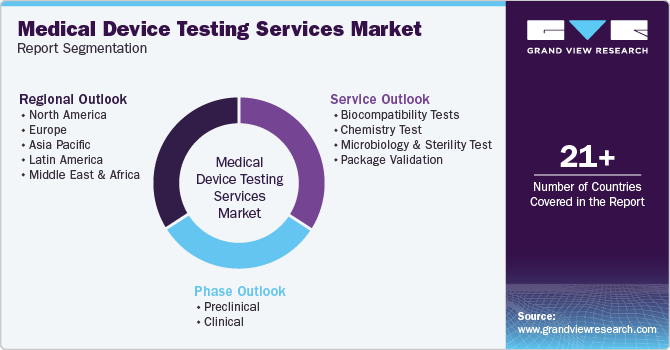
-
Service Outlook (Revenue, USD Billion, 2018 - 2030)
-
Biocompatibility Tests
-
Cardiovascular Device's Biocompatibility Tests
-
Orthopedic Device's Biocompatibility Tests
-
Dental Implant Devices' Biocompatibility Tests
-
Dermal Filler's Biocompatibility Tests
-
General Surgery Implantation Devices Biocompatibility Tests
-
Neurosurgical Implantation Devices Biocompatibility Tests
-
Ophthalmic Implantation Device's Biocompatibility Tests
-
Others
-
Chemistry Test
-
Chemical characterization (E&L)
-
Analytical method development and validation
-
Toxicological Risk Assessment and consulting
-
Microbiology & Sterility Test
-
Bioburden Determination
-
Pyrogen & Endotoxin Testing
-
Sterility Test & Validation
-
Antimicrobial Testing
-
Others
-
Package Validation
-
-
Phase Outlook (Revenue, USD Billion, 2018 - 2030)
-
Preclinical
-
Large animal research
-
Biocompatibility Tests
-
Chemistry Test
-
Microbiology & Sterility Test
-
-
Small animal research
-
Biocompatibility Tests
-
Chemistry Test
-
Microbiology & Sterility Test
-
-
-
Clinical
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
Netherlands
-
Belgium
-
Switzerland
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Malaysia
-
Indonesia
-
Singapore
-
Philippines
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
Colombia
-
Chile
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
Israel
-
Egypt
-
-
Frequently Asked Questions About This Report
b. The global medical device testing services market size was estimated at USD 8.1 billion in 2022 and is expected to reach USD 8.88 billion in 2023.
b. The global medical device testing services market is expected to grow at a compound annual growth rate of 9.0% from 2023 to 2030 to reach USD 16.29 billion by 2030.
b. The Asia Pacific dominated the medical device testing services market with a share of 41.0% in 2022. This is attributable to increasing R&D activities in this region and the growing adoption of new technologies in clinical trials.
b. Some key players operating in the medical device testing services market include SGS S.A., Labcorp (Toxikon, Inc), American Preclinical Services, Sterigenics International LLC, Charles River Laboratories, Element Minnetonka, North America Science Associates Inc., Eurofins Scientific, Pace Analytical Services LLC, Intertek Group Plc.
b. Key factors that are driving the medical device testing services market growth include increasing demand for in-vitro tests, recent technological advancements, and improving healthcare infrastructure in emerging economies.
Table of Contents
Chapter 1 Methodology and Scope
1.1 Market Segmentation & Scope
1.1.1 Regional Scope
1.1.2 Estimates And Forecast Timeline
1.2 Research Methodology
1.3 Information Procurement
1.3.1 Purchased Database
1.3.2 Gvr’s Internal Database
1.3.3 Secondary Sources
1.3.4 Primary Research
1.3.5 Details Of Primary Research
1.4 Information Or Data Analysis
1.4.1 Data Analysis Models
1.5 Market Formulation & Validation
1.5.1 Region Wise Market Calculation
1.5.2 Region Wise Market: Base Estimates
1.5.3 Global Market: Cagr Calculation
1.6 Model Details
1.6.1 Commodity Flow Analysis (Model 1)
1.6.2 Bottom-Up Approach (Model 2)
1.7 List Of Secondary Sources
1.8 List Of Primary Sources
1.9 List Of Abbreviations
1.10 Objectives
1.10.1 Objective - 1:
1.10.2 Objective - 2:
1.10.3 Objective - 3:
1.10.4 Objective - 4:
Chapter 2 Executive Summary
2.1 Market Snapshot
2.2 Segment Snapshot
2.3 Competitive Landscape Snapshot
Chapter 3 Medical Device Testing Services Market: Variables, Trends, & Scope
3.1 Market Lineage Outlook
3.1.1 Parent Market Outlook
3.1.1.1 Global Healthcare Cro Market
3.1.1.2 Global Medical Device Outsourcing Market
3.1.2 Ancillary Market Outlook
3.1.2.1 Global Pharmaceutical Analytical Testing Outsourcing Market
3.1.2.2 Global Bioanalytical Testing Services Market
3.2 User Perspective Analysis
3.2.1 Consumer Behavior Analysis
3.2.2 Market Influencer Analysis
3.3 Medical Device Testing Services Market Dynamics
3.3.1 Market Driver Analysis
3.3.1.1 Complexity In Product Design Of Medical Device Testing Services
3.3.1.2 Intense Competition In Medical Device Industry
3.3.1.3 Increasing Number Of Smaller Players Lacking In-House Testing Capabilities
3.3.1.4 Strict Approval Norms
3.3.2 Market Restraint Analysis
3.3.2.1 Legal And Regulatory Issues
3.3.2.2 Delays In Contractual Obligations
3.3.3 Industry Challenges
3.3.3.1 Managing Relationships
3.4 Medical Device Testing Services Market Analysis Tools
3.4.1 Porter's Five Forces Analysis
3.4.2 Pestel Analysis
3.5 Covid-19 Impact On Market
3.6 Impact Of Covid-19 On Regulatory Approvals In Eu
3.6.1 Postponement Of Eu-Mdr Applications
3.6.2 European Standards And Other Technical Documents Available For Free
3.6.3 Ppe & Relevant Medical Devices May Be Placed Without Ce Marking
3.6.4 Suspension Of Inspection By Nbs
3.7 Impact Of Covid-19 On Regulatory Approvals In The U.S.
3.8 Impact On Clinical Trials And Irb Reviews
3.9 Fda Asca Impact On Biocompatibility Testing
Chapter 4 Medical Device Testing Services Market: Service Segment Analysis
4.1 Medical Device Testing Services: Market By Molecule Segment: Key Takeaways
4.2 Medical Device Testing Services: Market Share Analysis, 2023 & 2030
4.3 Medical Device Testing Services Market Estimates & Forecast, By Service (USD Million)
4.4 Definitions & Scope
4.5 Biocompatibility Tests
4.5.1 Biocompatibility Medical Device Testing Services Market, 2018 - 2030 (USD Million)
4.5.2 Cardiovascular Device's Biocompatibility Tests
4.5.2.1 Cardiovascular Device's Biocompatibility Tests Market, 2018 - 2030 (USD Million)
4.5.3 Orthopedic Device's Biocompatibility Tests
4.5.3.1 Orthopedic Device's Biocompatibility Tests Market, 2018 - 2030 (USD Million)
4.5.4 Dental Implant Devices' Biocompatibility Tests
4.5.4.1 Dental Implant Devices' Biocompatibility Tests Market, 2018 - 2030 (USD Million)
4.5.5 Dermal Filler's Biocompatibility Tests
4.5.5.1 Dermal Filler's Biocompatibility Tests Market, 2018 - 2030 (USD Million)
4.5.6 General Surgery Implantation Devices Biocompatibility Tests
4.5.6.1 General Surgery Implantation Devices Biocompatibility Tests Market, 2018 - 2030 (USD Million)
4.5.7 Neurosurgical Implantation Devices Biocompatibility Tests
4.5.7.1 Neurosurgical Implantation Devices Biocompatibility Tests Market, 2018 - 2030 (USD Million)
4.5.8 Ophthalmic Implantation Device's Biocompatibility Tests
4.5.8.1 Ophthalmic Implantation Device's Biocompatibility Tests Market, 2018 - 2030 (USD Million)
4.5.9 Others
4.5.9.1 Others Biocompatibility Test Market, 2018 - 2030 (USD Million)
4.6 Chemistry Test
4.6.1 Chemistry Medical Device Testing Services Market, 2018 - 2030 (USD Million)
4.6.2 Chemical Characterization (E&L)
4.6.2.1 Chemical Characterization (E&L) Market, 2018 - 2030 (USD Million)
4.6.3 Analytical Method Development And Validation
4.6.3.1 Analytical Method Development And Validation Market, 2018 - 2030 (USD Million)
4.6.4 Toxicological Risk Assessment And Consulting
4.6.4.1 Toxicological Risk Assessment And Consulting Market, 2018 - 2030 (USD Million)
4.7 Microbiology & Sterility Testing
4.7.1 Microbiology & Sterility Medical Device Testing Services Market, 2018 - 2030 (USD Million)
4.7.2 Bioburden Determination
4.7.2.1 Bioburden Determination Market, 2018 - 2030 (USD Million)
4.7.3 Pyrogen & Endotoxin Testing
4.7.3.1 Pyrogen & Endotoxin Testing Market, 2018 - 2030 (USD Million)
4.7.4 Sterility Test & Validation
4.7.4.1 Sterility Test & Validation Market, 2018 - 2030 (USD Million)
4.7.5 Antimicrobial Testing
4.7.5.1 Antimicrobial Testing Market, 2018 - 2030 (USD Million)
4.7.6 Others Microbiology & Sterility Test
4.7.6.1 Others Microbiology & Sterility Test Market, 2018 - 2030 (USD Million)
4.8 Package Validation
4.8.1 Package Validation Medical Device Testing Services Market, 2018 - 2030 (USD Million)
Chapter 5 Medical Device Testing Services Market: Phase And Service Criss-Cross Segment Analysis
5.1 Medical Device Testing Services: Market By Phase And Service Criss-Cross Segment: Key Takeaways
5.2 Medical Device Testing Services: Market Share Analysis, 2023 & 2030
5.3 Medical Device Testing Services Market Estimates & Forecast, By Phase And Service Criss-Cross (USD Million)
5.4 Definitions & Scope
5.5 Preclinical
5.5.1 Preclinical Market, 2018 - 2030 (USD Million)
5.5.2 Small Animal Research
5.5.2.1 Small Animal Research Market, 2018 - 2030 (USD Million)
5.5.2.2 Biocompatibility Tests
5.5.2.2.1 Biocompatibility Tests Market, 2018 - 2030 (USD Million)
5.5.2.3 Chemistry Test
5.5.2.3.1 Chemistry Test Market, 2018 - 2030 (USD Million)
5.5.2.4 Microbiology & Sterility Test
5.5.2.4.1 Microbiology & Sterility Test Market, 2018 - 2030 (USD Million)
5.5.3 Large Animal Research
5.5.3.1 Large Animal Research Market, 2018 - 2030 (USD Million)
5.5.3.2 Biocompatibility Tests
5.5.3.2.1 Biocompatibility Tests Market, 2018 - 2030 (USD Million)
5.5.3.3 Chemistry Test
5.5.3.3.1 Chemistry Test Market, 2018 - 2030 (USD Million)
5.5.3.4 Microbiology & Sterility Test
5.5.3.4.1 Microbiology & Sterility Test Market, 2018 - 2030 (USD Million)
5.5.4 Others
5.5.4.1 Others Market, 2018 - 2030 (USD Million)
5.6 Clinical
5.6.1.1 Clinical Market, 2018 - 2030 (USD Million)
Chapter 6 Medical Device Testing Services Market: Regional Analysis
6.1 Medical Device Testing Services: Market Share Analysis, 2023 & 2030
6.2 Medical Device Testing Services: Market Estimates And Forecast, By Region (USD Million)
6.3 North America
6.3.1 Competitive Scenario
6.3.2 North America Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.3.3 U.S.
6.3.3.1 Key Country Dynamics
6.3.3.2 Competitive Scenario
6.3.3.3 Regulatory Scenario
6.3.3.4 U.S. Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.3.4 Canada
6.3.4.1 Key Country Dynamics
6.3.4.2 Competitive Scenario
6.3.4.3 Regulatory Scenario
6.3.4.4 Canada Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.4 Europe
6.4.1 Competitive Scenario
6.4.2 Europe Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.4.3 Uk
6.4.3.1 Key Country Dynamics
6.4.3.2 Competitive Scenario
6.4.3.3 Regulatory Scenario
6.4.3.4 Uk Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.4.4 Germany
6.4.4.1 Key Country Dynamics
6.4.4.2 Competitive Scenario
6.4.4.3 Regulatory Scenario
6.4.4.4 Germany Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.4.5 France
6.4.5.1 Key Country Dynamics
6.4.5.2 Competitive Scenario
6.4.5.3 Regulatory Scenario
6.4.5.4 Device Classification
6.4.5.5 France Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.4.6 Italy
6.4.6.1 Key Country Dynamics
6.4.6.2 Competitive Scenario
6.4.6.3 Regulatory Scenario
6.4.6.4 Italy Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.4.7 Spain
6.4.7.1 Key Country Dynamics
6.4.7.2 Competitive Scenario
6.4.7.3 Regulatory Scenario
6.4.7.4 Spain Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.4.8 Denmark
6.4.8.1 Key Country Dynamics
6.4.8.2 Competitive Scenario
6.4.8.3 Regulatory Scenario
6.4.8.4 Denmark Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.4.9 Sweden
6.4.9.1 Key Country Dynamics
6.4.9.2 Competitive Scenario
6.4.9.3 Regulatory Scenario
6.4.9.4 Sweden Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.4.10 Norway
6.4.10.1 Key Country Dynamics
6.4.10.2 Competitive Scenario
6.4.10.3 Regulatory Scenario
6.4.10.4 Norway Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.4.11 Netherlands
6.4.11.1 Key Country Dynamics
6.4.11.2 Competitive Scenario
6.4.11.3 Regulatory Scenario
6.4.11.4 Netherlands Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.4.12 Belgium
6.4.12.1 Competitive Scenario
6.4.12.2 Regulatory Scenario
6.4.12.3 Belgium Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.4.13 Switzerland
6.4.13.1 Key Country Dynamics
6.4.13.2 Competitive Scenario
6.4.13.3 Regulatory Scenario
6.4.13.4 Switzerland Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.5 Asia Pacific
6.5.1 Competitive Scenario
6.5.2 Asia Pacific Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.3 Japan
6.5.3.1 Key Country Dynamics
6.5.3.2 Competitive Scenario
6.5.3.3 Regulatory Scenario
6.5.3.4 Japan Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.4 China
6.5.4.1 Key Country Dynamics
6.5.4.2 Competitive Scenario
6.5.4.3 Regulatory Scenario
6.5.4.4 China Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.5 India
6.5.5.1 Key Country Dynamics
6.5.5.2 Competitive Scenario
6.5.5.3 Regulatory Scenario
6.5.5.4 India Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.6 Australia
6.5.6.1 Key Country Dynamics
6.5.6.2 Competitive Scenario
6.5.6.3 Regulatory Scenario
6.5.6.4 Australia Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.7 South Korea
6.5.7.1 Key Country Dynamics
6.5.7.2 Competitive Scenario
6.5.7.3 Regulatory Scenario
6.5.7.4 South Korea Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.8 Malaysia
6.5.8.1 Key Country Dynamics
6.5.8.2 Competitive Scenario
6.5.8.3 Regulatory Scenario
6.5.8.4 Malaysia Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.9 Indonesia
6.5.9.1 Key Country Dynamics
6.5.9.2 Competitive Scenario
6.5.9.3 Regulatory Scenario
6.5.9.4 Indonesia Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.10 Singapore
6.5.10.1 Key Country Dynamics
6.5.10.2 Competitive Scenario
6.5.10.3 Regulatory Scenario
6.5.10.4 Singapore Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.11 Philippines
6.5.11.1 Competitive Scenario
6.5.11.2 Regulatory Scenario
6.5.11.3 Philippines Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.5.12 Thailand
6.5.12.1 Key Country Dynamics
6.5.12.2 Competitive Scenario
6.5.12.3 Regulatory Scenario
6.5.12.4 Thailand Medical Device Testing Services Market, 2018 - 2030 (USD Million)
6.6 Latin America
6.6.1 Competitive Scenario
6.6.2 Latin America Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030 (USD Million)
6.6.3 Brazil
6.6.3.1 Key Country Dynamics
6.6.3.2 Competitive Scenario
6.6.3.3 Regulatory Scenario
6.6.3.4 Brazil Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030 (USD Million)
6.6.4 Mexico
6.6.4.1 Key Country Dynamics
6.6.4.2 Competitive Scenario
6.6.4.3 Regulatory Scenario
6.6.4.3.1 Medical Device
6.6.4.4 Mexico Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030 (USD Million)
6.6.5 Argentina
6.6.5.1 Key Country Dynamics
6.6.5.2 Competitive Scenario
6.6.5.3 Regulatory Scenario
6.6.5.4 Argentina Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.6.6 Colombia
6.6.6.1 Key Country Dynamics
6.6.6.2 Competitive Scenario
6.6.6.3 Regulatory Scenario
6.6.6.4 Colombia Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.6.7 Chile
6.6.7.1 Competitive Scenario
6.6.7.2 Regulatory Scenario
6.6.7.3 Chile Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.7 MEA
6.7.1 Competitive Scenario
6.7.2 MEA Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.7.3 South Africa
6.7.3.1 Key Country Dynamics
6.7.3.2 Competitive Scenario
6.7.3.3 Regulatory Scenario
6.7.3.4 South Africa Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.7.4 Saudi Arabia
6.7.4.1 Key Country Dynamics
6.7.4.2 Competitive Scenario
6.7.4.3 Regulatory Scenario
6.7.4.4 Saudi Arabia Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030, (USD Million)
6.7.5 UAE
6.7.5.1 Key Country Dynamics
6.7.5.2 Competitive Scenario
6.7.5.3 Regulatory Scenario
6.7.5.4 Uae Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030 (USD Million)
6.7.6 Kuwait
6.7.6.1 Key Country Dynamics
6.7.6.2 Competitive Scenario
6.7.6.3 Regulatory Scenario
6.7.6.4 Kuwait Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030 (USD Million)
6.7.7 Israel
6.7.7.1 Key Country Dynamics
6.7.7.2 Competitive Scenario
6.7.7.3 Regulatory Scenario
6.7.7.4 Israel Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030 (USD Million)
6.7.8 Egypt
6.7.8.1 Competitive Scenario
6.7.8.2 Regulatory Scenario
6.7.8.3 Egypt Medical Device Testing Services Market Estimates And Forecasts, 2018 - 2030 (USD Million)
Chapter 7 Competitive Landscape
7.1. Company Landscape
7.1.1. SGS SA
7.1.1.1. Company overview
7.1.1.2. Financial performance
7.1.1.3. Product benchmarking
7.1.1.4. Strategic initiatives
7.1.2. Laboratory Corporation of America Holdings
7.1.2.1. Company overview
7.1.2.2. Financial performance
7.1.2.3. Product benchmarking
7.1.2.4. Strategic initiatives
7.1.3. Nelson Laboratories, LLC
7.1.3.1. Company overview
7.1.3.2. Financial performance
7.1.3.3. Product benchmarking
7.1.3.4. Strategic initiatives
7.1.4. TÜV SÜD
7.1.4.1. Company overview
7.1.4.2. Financial performance
7.1.4.3. Product benchmarking
7.1.4.4. Strategic initiatives
7.1.5. Charles River Laboratories
7.1.5.1. Company overview
7.1.5.2. Financial performance
7.1.5.3. Product benchmarking
7.1.5.4. Strategic initiatives
7.1.6. Element Minnetonka
7.1.6.1. Company overview
7.1.6.2. Financial performance
7.1.6.3. Product benchmarking
7.1.6.4. Strategic initiatives
7.1.7. North America Science Associates Inc. (NAMSA)
7.1.7.1. Company overview
7.1.7.2. Financial performance
7.1.7.3. Product benchmarking
7.1.7.4. Strategic initiatives
7.1.8. Eurofins Scientific
7.1.8.1. Company overview
7.1.8.2. Financial performance
7.1.8.3. Product benchmarking
7.1.8.4. Strategic initiatives
7.1.9. Pace Analytical Services LLC
7.1.9.1. Company overview
7.1.9.2. Financial performance
7.1.9.3. Product benchmarking
7.1.9.4. Strategic initiatives
7.1.10. Intertek Group Plc
7.1.10.1. Company overview
7.1.10.2. Financial performance
7.1.10.3. Product benchmarking
7.1.10.4. Strategic initiatives
7.1.11. WuXi AppTec
7.1.11.1. Company overview
7.1.11.2. Financial performance
7.1.11.3. Product benchmarking
7.1.11.4. Strategic initiatives
7.2.4 Participant Categorization
7.2.1 Market Leaders
7.2.1.1 Medical Device Testing Services Market Share Analysis, 2023
7.3 Strategy Mapping
7.3.1 Service Launches/Upgrades
7.3.2 Merger/Acquisitions/Joint Ventures Merger
7.3.3 Partnership/Agreements
7.3.4 Expansions
7.3.5 Others
7.4 Market Position Analysis, 2022/2023 (Heat Map Analysis)
Chapter 8 Key Takeaways
List of Tables
Table 1 List of abbreviation
Table 2 North America medical device testing services market, by region, 2018 - 2030 (USD Million)
Table 3 North America medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 4 North America medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 5 U.S. medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 6 U.S. medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 7 Canada medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 8 Canada medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 9 Europe medical device testing services market, by region, 2018 - 2030 (USD Million)
Table 10 Europe medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 11 Europe medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 12 UK medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 13 UK medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 14 Germany medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 15 Germany medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 16 France medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 17 France medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 18 Italy medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 19 Italy medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 20 Spain medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 21 Spain medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 22 Denmark medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 23 Denmark medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 24 Sweden medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 25 Sweden medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 26 Norway medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 27 Norway medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 28 Netherlands medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 29 Netherlands medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 30 Belgium medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 31 Belgium medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 32 Asia Pacific medical device testing services market, by region, 2018 - 2030 (USD Million)
Table 33 Asia Pacific medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 34 Asia Pacific medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 35 China medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 36 China medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 37 Japan medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 38 Japan medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 39 India medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 40 India medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 41 Australia medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 42 Australia medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 43 South Korea medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 44 South Korea medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 45 Malaysia medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 46 Malaysia medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 47 Indonesia medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 48 Indonesia medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 49 Singapore medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 50 Singapore medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 51 Philippines medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 52 Philippines medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 53 Thailand medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 54 Thailand medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 55 Latin America medical device testing services market, by region, 2018 - 2030 (USD Million)
Table 56 Latin America medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 57 Latin America medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 58 Brazil medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 59 Brazil medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 60 Mexico medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 61 Mexico medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 62 Argentina medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 63 Argentina medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 64 Colombia medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 65 Colombia medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 66 Chile medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 67 Chile medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 68 MEA medical device testing services market, by region, 2018 - 2030 (USD Million)
Table 69 MEA medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 70 MEA medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 71 South Africa medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 72 South Africa medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 73 Saudi Arabia medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 74 Saudi Arabia medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 75 UAE medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 76 UAE medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 77 Kuwait medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 78 Kuwait medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 79 Israel medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 80 Israel medical device testing services market, by phase, 2018 - 2030 (USD Million)
Table 81 Egypt medical device testing services market, by service, 2018 - 2030 (USD Million)
Table 82 Egypt medical device testing services market, by phase, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Medical device testing services market segmentation
Fig. 2 Market research process
Fig. 3 Information procurement
Fig. 4 Primary research pattern
Fig. 5 Market research approaches
Fig. 6 QFD modeling for market share assessment
Fig. 7 Market formulation & validation
Fig. 8 Commodity flow analysis
Fig. 9 Bottom-up approach
Fig. 10 Market snapshot
Fig. 11 Segment snapshot
Fig. 12 Competitive landscape snapshot
Fig. 13 Parent market outlook, 2023 (USD Billion)
Fig. 14 Ancillary market outlook, 2023 (USD Billion)
Fig. 15 Medical device testing services market dynamics
Fig. 16 Porter's five forces analysis
Fig. 17 SWOT analysis, by factor (political & legal, economic, and technological)
Fig. 18 List of FDA-recognized consensus test methods in the ASCA pilot for biocompatibility testing of medical devices
Fig. 19 Medical Device Testing Services market molecule outlook: Segment dashboard
Fig. 20 Medical Device Testing Services market: Molecule movement analysis
Fig. 21 Biocompatibility medical device testing services market, 2018 - 2030 (USD Million)
Fig. 22 Cardiovascular device’s biocompatibility tests market, 2018 - 2030 (USD Million)
Fig. 23 Orthopedic device's biocompatibility tests market, 2018 - 2030 (USD Million)
Fig. 24 Dental implant devices' biocompatibility tests market, 2018 - 2030 (USD Million)
Fig. 25 Dermal filler's biocompatibility tests market, 2018 - 2030 (USD Million)
Fig. 26 General surgery implantation devices biocompatibility tests market, 2018 - 2030 (USD Million)
Fig. 27 Neurosurgical implantation devices biocompatibility tests market, 2018 - 2030 (USD Million)
Fig. 28 Ophthalmic implantation device's biocompatibility tests market, 2018 - 2030 (USD Million)
Fig. 29 Others Biocompatibility test market, 2018 - 2030 (USD Million)
Fig. 30 Chemistry medical device testing services market, 2018 - 2030 (USD Million)
Fig. 31 Chemical characterization (E&L) market, 2018 - 2030 (USD Million)
Fig. 32 Analytical method development and validation market, 2018 - 2030 (USD Million)
Fig. 33 Toxicological risk assessment and consulting market, 2018 - 2030 (USD Million)
Fig. 34 Microbiology & sterility medical device testing services market, 2018 - 2030 (USD Million)
Fig. 35 Bioburden determination market, 2018 - 2030 (USD Million)
Fig. 36 Pyrogen & endotoxin testing market, 2018 - 2030 (USD Million)
Fig. 37 Sterility test & validation market, 2018 - 2030 (USD Million)
Fig. 38 Antimicrobial testing market, 2018 - 2030 (USD Million)
Fig. 39 Others microbiology & sterility test market, 2018 - 2030 (USD Million)
Fig. 40 Package validation medical device testing services market, 2018 - 2030 (USD Million)
Fig. 41 Medical device testing services market phase and service criss-cross outlook: Segment dashboard
Fig. 42 Medical device testing services market: Phase and Service Criss-Cross movement analysis
Fig. 43 Preclinical market, 2018 - 2030 (USD Million)
Fig. 44 Small animal research market, 2018 - 2030 (USD Million)
Fig. 45 Biocompatibility Tests market, 2018 - 2030 (USD Million)
Fig. 46 Chemistry test market, 2018 - 2030 (USD Million)
Fig. 47 Microbiology & sterility test market, 2018 - 2030 (USD Million)
Fig. 48 Large animal research market, 2018 - 2030 (USD Million)
Fig. 49 Biocompatibility Tests market, 2018 - 2030 (USD Million)
Fig. 50 Chemistry test market, 2018 - 2030 (USD Million)
Fig. 51 Microbiology & sterility test market, 2018 - 2030 (USD Million)
Fig. 52 Others market, 2018 - 2030 (USD Million)
Fig. 53 Clinical market, 2018 - 2030 (USD Million)
Fig. 54 Regional market: Key takeaways
Fig. 55 Regional outlook, 2023 & 2030
Fig. 56 Regional outlook, 2023 & 2030
Fig. 57 North America market, 2018 - 2030 (USD Million)
Fig. 58 Key country dynamics
Fig. 59 U.S. Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 60 Key country dynamics
Fig. 61 Canada Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 62 Europe Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 63 Key country dynamics
Fig. 64 UK Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 65 Key country dynamics
Fig. 66 Germany Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 67 Key country dynamics
Fig. 68 France medical device classification
Fig. 69 France Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 70 Key country dynamics
Fig. 71 Italy Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 72 Key country dynamics
Fig. 73 Spain Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 74 Key country dynamics
Fig. 75 Denmark Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 76 Key country dynamics
Fig. 77 Sweden Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 78 Key country dynamics
Fig. 79 Norway Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 80 Key country dynamics
Fig. 81 Netherlands Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 82 Belgium Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 83 Key country dynamics
Fig. 84 Switzerland Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 85 Asia Pacific Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 86 Key country dynamics
Fig. 87 Japan Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 88 Key country dynamics
Fig. 89 China Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 90 Key country dynamics
Fig. 91 India Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 92 Key country dynamics
Fig. 93 Australia Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 94 Key country dynamics
Fig. 95 South Korea Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 96 Key country dynamics
Fig. 97 Malaysia Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 98 Key country dynamics
Fig. 99 Indonesia Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 100 Key country dynamics
Fig. 101 Singapore Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 102 Philippines Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 103 Key country dynamics
Fig. 104 Thailand Medical Device Testing Services Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 105 Latin America Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 106 Key country dynamics
Fig. 107 Brazil Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 108 Key country dynamics
Fig. 109 Mexico Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 110 Key country dynamics
Fig. 111 Argentina Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 112 Key country dynamics
Fig. 113 Colombia Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 114 Chile Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 115 MEA Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 116 Key country dynamics
Fig. 117 South Africa Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 118 Key country dynamics
Fig. 119 Saudi Arabia Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 120 Key country dynamics
Fig. 121 UAE Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 122 Key country dynamics
Fig. 123 Kuwait Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 124 Key country dynamics
Fig. 125 Israel Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 126 Egypt Medical Device Testing Services market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 127 Market participant categorization
Fig. 128 Market participant categorization
Fig. 129 Heat map analysisWhat questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Medical Device Testing Services market Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Medical Device Testing Services market Phase Outlook (Revenue in USD Million, 2018 - 2030)Preclinical
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Medical Device Testing Services market Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- North America Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- North America Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- U.S.
- U.S. Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- U.S. Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- U.S. Service Outlook (Revenue in USD Million, 2018 - 2030)
- Canada
- Canada Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Canada Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Canada Service Outlook (Revenue in USD Million, 2018 - 2030)
- North America Service Outlook (Revenue in USD Million, 2018 - 2030)
- Europe
- Europe Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Europe Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- UK
- UK Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- UK Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- UK Service Outlook (Revenue in USD Million, 2018 - 2030)
- Germany
- Germany Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Germany Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Germany Service Outlook (Revenue in USD Million, 2018 - 2030)
- France
- France Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- France Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- France Service Outlook (Revenue in USD Million, 2018 - 2030)
- Italy
- Italy Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Italy Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Italy Service Outlook (Revenue in USD Million, 2018 - 2030)
- Spain
- Spain Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Spain Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Spain Service Outlook (Revenue in USD Million, 2018 - 2030)
- Denmark
- Denmark Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Denmark Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Denmark Service Outlook (Revenue in USD Million, 2018 - 2030)
- Sweden
- Sweden Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Sweden Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Sweden Service Outlook (Revenue in USD Million, 2018 - 2030)
- Norway
- Norway Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Norway Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Norway Service Outlook (Revenue in USD Million, 2018 - 2030)
- Netherlands
- Netherlands Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Netherlands Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Netherlands Service Outlook (Revenue in USD Million, 2018 - 2030)
- Belgium
- Belgium Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Belgium Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Belgium Service Outlook (Revenue in USD Million, 2018 - 2030)
- Switzerland
- Switzerland Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Switzerland Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Switzerland Service Outlook (Revenue in USD Million, 2018 - 2030)
- Europe Service Outlook (Revenue in USD Million, 2018 - 2030)
- Asia Pacific
- Asia Pacific Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Asia Pacific Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Japan
- Japan Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Japan Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Japan Service Outlook (Revenue in USD Million, 2018 - 2030)
- China
- China Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- China Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- China Service Outlook (Revenue in USD Million, 2018 - 2030)
- India
- India Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- India Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- India Service Outlook (Revenue in USD Million, 2018 - 2030)
- Australia
- Australia Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Australia Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Australia Service Outlook (Revenue in USD Million, 2018 - 2030)
- South Korea
- South Korea Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- South Korea Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- South Korea Service Outlook (Revenue in USD Million, 2018 - 2030)
- Malaysia
- Malaysia Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Malaysia Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Malaysia Service Outlook (Revenue in USD Million, 2018 - 2030)
- Indonesia
- Indonesia Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Indonesia Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Indonesia Service Outlook (Revenue in USD Million, 2018 - 2030)
- Singapore
- Singapore Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Singapore Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Singapore Service Outlook (Revenue in USD Million, 2018 - 2030)
- Philippines
- Philippines Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Philippines Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Philippines Service Outlook (Revenue in USD Million, 2018 - 2030)
- Thailand
- Thailand Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Thailand Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Thailand Service Outlook (Revenue in USD Million, 2018 - 2030)
- Asia Pacific Service Outlook (Revenue in USD Million, 2018 - 2030)
- Latin America
- Latin America Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Latin America Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Brazil
- Brazil Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Brazil Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Brazil Service Outlook (Revenue in USD Million, 2018 - 2030)
- Mexico
- Mexico Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Mexico Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Mexico Service Outlook (Revenue in USD Million, 2018 - 2030)
- Argentina
- Argentina Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Argentina Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Argentina Service Outlook (Revenue in USD Million, 2018 - 2030)
- Colombia
- Colombia Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Colombia Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Colombia Service Outlook (Revenue in USD Million, 2018 - 2030)
- Chile
- Chile Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Chile Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Chile Service Outlook (Revenue in USD Million, 2018 - 2030)
- Latin America Service Outlook (Revenue in USD Million, 2018 - 2030)
- Middle East and Africa (MEA)
- MEA Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- MEA Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- South Africa
- South Africa Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- South Africa Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- South Africa Service Outlook (Revenue in USD Million, 2018 - 2030)
- Saudi Arabia
- Saudi Arabia Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Saudi Arabia Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Saudi Arabia Service Outlook (Revenue in USD Million, 2018 - 2030)
- UAE
- UAE Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- UAE Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- UAE Service Outlook (Revenue in USD Million, 2018 - 2030)
- Kuwait
- Kuwait Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Kuwait Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Kuwait Service Outlook (Revenue in USD Million, 2018 - 2030)
- Israel
- Israel Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Israel Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Israel Service Outlook (Revenue in USD Million, 2018 - 2030)
- Egypt
- Egypt Service Outlook (Revenue in USD Million, 2018 - 2030)
- Biocompatibility Tests
- Cardiovascular Device's Biocompatibility Tests
- Orthopedic Device's Biocompatibility Tests
- Dental Implant Devices' Biocompatibility Tests
- Dermal Filler's Biocompatibility Tests
- General Surgery Implantation Devices Biocompatibility Tests
- Neurosurgical Implantation Devices Biocompatibility Tests
- Ophthalmic Implantation Device's Biocompatibility Tests
- Others
- Chemistry Test
- Chemical characterization (E&L)
- Analytical method development and validation
- Toxicological Risk Assessment and consulting
- Microbiology & Sterility Test
- Bioburden Determination
- Pyrogen & Endotoxin Testing
- Sterility Test & Validation
- Antimicrobial Testing
- Others
- Package Validation
- Biocompatibility Tests
- Egypt Phase Outlook (Revenue in USD Million, 2018 - 2030)
- Large animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Small animal research
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Test
- Clinical
- Large animal research
- Egypt Service Outlook (Revenue in USD Million, 2018 - 2030)
- MEA Service Outlook (Revenue in USD Million, 2018 - 2030)
- North America
Medical Device Testing Services Market Dynamics
Driver: Complexity in Product Design
Complex designs that require comprehensive and specialized testing elevate the need for multifaceted assessments, driving the demand for testing services. Testing labs and service providers experience heightened demand for diverse and advanced testing procedures, leading to market expansion. The emergence of personalized medicine, drug-device combinations, artificial intelligence, wearables, and increased focus on real-time patient monitoring resulted in a complex medical device ecosystem. Real-time patient monitoring, remote patient monitoring, and continuous patient monitoring are the focal areas of interest in chronic disease management, such as diabetes & cardiovascular diseases. In this complex ecosystem, medical devices must be accurate, durable, and easy to operate. To match these standards, devices must undergo shear testing in accelerated conditions to obtain marketing approval and have a competitive advantage. Conducting in-house tests is a matter of time, labor, and cost; outsourcing these tests helps overcome their limitations.
Driver: Intense Competition in Medical Device Industry
The medical device industry is witnessing fierce competition where companies use offensive and defensive marketing strategies to sustain short- & long-term competition. Key strategies include new product launches, extensive R&D, competitive pricing, collaborative development, regional expansion, and mergers & acquisitions. Moreover, intense competition necessitates rapid product development and market entry. Medical device testing services are crucial in expediting the regulatory approval process. Considering that the approval processes for medical devices are not simple, companies need a sound knowledge of updated regulatory norms and protocols. Not all companies have such expertise; hence, outsourcing it to a specialized firm is a wise option for medical device companies. Thus, service providers offering efficient and timely testing contribute to reducing time-to-market for medical devices and offer companies a competitive advantage.
Restraint: Legal and Regulatory Issues
There are a large number of legal issues in offshore outsourcing to emerging economies, such as India and China. If the medical devices are outsourced to other nations, service providers are expected to be aware of data privacy & Intellectual Property protection in the concerned country. Outsourcing has raised various cross-border legal issues that have received attention from government and private groups. One of the most important issues is the increasing burden on outsourcing service providers and customers to protect information. Most of the outsourcing in the medical device industry is driven by companies wanting to curb their costs. However, the desire to cut costs is not the only reason; there are safety regulations of various nations that are a major concern; this is one of the restraints of the medical device outsourcing market. Furthermore, ethical considerations, data security, and privacy compliance-especially under regulations, such as GDPR-can restrict market growth. Upholding ethical standards, ensuring data security, and maintaining compliance with quality assurance protocols, such as ISO 17025, are crucial for credibility.
What Does This Report Include?
This section will provide insights into the contents included in this medical device testing services market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Medical device testing services market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Medical device testing services market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the medical device testing services market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for medical device testing services market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of medical device testing services market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Medical Device Testing Services Market Categorization:
The medical device testing services market was categorized into three segments, namely service (Biocompatibility Tests, Chemistry Test, Microbiology & Sterility Testing, Package Validation), phase (Preclinical, Clinical), and region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa)
Segment Market Methodology:
The medical device testing services market was segmented into service, phase, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The medical device testing services market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into thirty-five countries, namely, the U.S.; Canada; the UK; Germany; France; Italy; Spain; Netherlands; Belgium; Switzerland; Russia; Sweden; Denmark; Norway; Japan; China; India; Australia; South Korea; Malaysia; Indonesia; Singapore; Philippines; Thailand; Brazil; Mexico; Argentina; Colombia; Chile; South Africa; Saudi Arabia; UAE; Israel; Egypt; Kuwait.
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
Medical device testing services market companies & financials:
The medical device testing services market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
SGS SA (SGS Société Générale de Surveillance SA) - SGS SA (SGS) provides clinical research, quality assurance, inspection, testing, and certification services worldwide. It operates through nine reportable segments: agriculture, food & life, minerals, oil, gas & chemicals, consumer retail, certification & business enhancement, industrial, environment, health & safety, transportation, and governments & institutions. Under clinical research services, it offers clinical development consultancy, pharmacovigilance & drug safety services, and early-phase clinical services (patent & clinical trials and bioequivalence & bioavailability studies). It also provides verification & certification services to enable its customer’s products to be in line with international standards, outsourcing, consulting, and data analytic services. Moreover, SGS caters to its services through a network of 2,600 offices and laboratories.
-
Laboratory Corporation of America Holdings - It is a global life sciences company. Through its Labcorp Drug Development (DD) and Labcorp Diagnostics (Dx) segments, the company provides drug development, diagnostic, & technology-enabled solutions for over 160 million patient encounters annually. Its key services include analysis, research models, lead optimization, pharmaceutical consulting, clinical testing & development, and commercialization. With over 80,000 employees, the company offers its products in over 100 countries. In addition, the company supports clinical trial activity with its preclinical & clinical development and central laboratory businesses.
-
Nelson Laboratories, LLC - Sterigenics International LLC is a contract sterilization services company. The company has a global network of 46 facilities. Its three operating companies are Nelson Labs, Nordion, and Sterigenics. In addition, it has multiple certifications, such as ISO 13485:2003 & EN ISO 13485:2012 and ISO 9001:2008, and Eudralex Good Manufacturing Practice (GMP) regulatory certificate. In 2016, the company acquired Nelson Laboratories, a laboratory testing and consulting services company.
-
TÜV SÜD - TUV SUD A.G. is a technical service organization present in 850 locations across the globe. The company has an extensive network of accredited laboratories, offices, and multidisciplinary experts. It operates through four segments: industry, certification, mobility, and others. The services offered by the company are auditing & system certification, inspection, global market access, knowledge services, product certification, testing, and training.
-
Charles River Laboratories - Charles River Laboratories is a global provider of products and services to support the drug discovery, development, & safety testing processes for pharmaceutical, biotechnology, & other life sciences companies. It operates through three segments: discovery & safety assessment, research models & services, and manufacturing support. The company serves customers in over 100 countries and has facilities in North America, Europe, and Asia Pacific.
-
Element Minnetonka - Element Minnetonka, formerly Medical Devices Testing Services, offers various analytical testing services for medical device companies. The services include consulting, mechanical testing, characterization, inspection, compliance with standards, and maintenance of instruments & fixtures. The company’s tests are compliant with regulatory bodies, such as the U.S. FDA and Conformité Européenne (CE), and it also has certifications from the International Standardization Organization (ISO) and the American Society for Testing & Materials (ASTM). In 2017, the company was acquired by Element Materials Technology.
-
North America Science Associates LLC (NAMSA) - North American Science Associates, LCC (NAMSA) is the provider of regulatory consulting, laboratory testing, and compliance services. The company offers various services, such as medical device testing, regulatory, reimbursement & quality consulting, and clinical research services. It also provides sterilization products, such as biological & chemical indicators, and test suspensions. In addition, it offers NAMSA Test Navigator, an online test selection tool. It also caters its services through network of distributors & sales representatives in the U.S. and across the globe.
-
Eurofins Scientific - Eurofins Scientific SE (Eurofins) is a provider of preclinical services across the globe. It has a portfolio of 150,000 analytical methods for evaluating the composition, authenticity, and purity of biological substances. It also provides AgroTesting, consumer product testing, environment testing, medical device testing, and chemical registration & authorization services. Eurofins caters its portfolio of services to pharmaceutical, consumer products, food, and the environment with a network of 375 laboratories in 44 countries.
-
Pace Analytical Services, LLC - It offers chemistry services, such as method development, validation, verification, and method transfer services, including raw material testing, extractable & leachable testing, quality testing, residual solvents testing, environment monitoring, impurities testing, and microbiology laboratory testing. It also provides other services, such as scientific staffing, instrument sales & services, and consulting.
-
Intertek Group Plc - Intertek Group plc (IGP) provides quality and safety solutions. The company operates through three reportable segments: product, trade, and resources. Its product segment’s key offerings include biopharmaceutical services, protein structure analysis, impurity testing, inspection & certification services, laboratory testing, quality & performance testing, and sustainability analysis. Its trade and resources segment offers technical services, analytical testing services, calibration, and asset integrity services. IGP caters to life sciences, food, pharmaceuticals, and healthcare industries. It has a presence in 1,000 locations in over 100 countries.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Medical Device Testing Services Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2022, historic information from 2018 to 2021, and forecast from 2023 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Medical Device Testing Services Market Report Assumptions:
-
The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





