Minimally Invasive Thoracic Surgery Market Summary
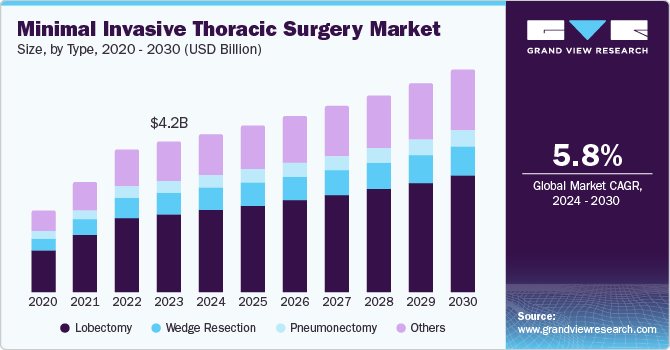
The global minimally invasive thoracic surgery market size was estimated at USD 4.22 billion in 2023 and is projected to reach USD 6.24 billion by 2030, growing at a CAGR of 5.8% from 2024 to 2030. The increasing number of lung cancer cases is propelling the demand for improved patient care, leading to the growth and transformation of the market.
Key Market Trends & Insights
- The North America captured nearly 50% of the global market in 2023 due to several key factors.
- The U.S. Minimally Invasive Thoracic Surgery market is expected to grow significantly from 2024 to 2030.
- Based on types, the lobectomy segment accounted for 51.9% of the total revenue in 2023.
- Based on types, the wedge resection segment is projected to grow at a CAGR of 4.8% from 2024 to 2030.
Market Size & Forecast
- 2023 Market Size: USD 4.22 Billion
- 2030 Projected Market USD 6.24 Billion
- CAGR (2024-2030): 5.8%
- North America: Largest market in 2023
This growth is also driven by evolving patient preferences, which necessitate better accommodation, daily routines, check-ups, and improved patient-hospital staff relationships.
According to a survey by the American Cancer Society, lung cancer accounts for approximately one in five cancer-related deaths worldwide. Advances in surgical techniques have made it possible to prevent it, leading to an increased demand for minimally invasive thoracic surgeries.
The minimally invasive thoracic surgery market is seeing rapid advancements due to the continuous development of new technologies, such as Da Vinci surgical instruments used in laparoscopic surgery. Advancing health insurance companies that help cover patients' expenses has helped patients receive surgery treatment and increased the market's growth.
Types Insights
The lobectomy segment accounted for 51.9% of the total revenue in 2023. The high rate of lobectomy procedures can be linked to the rising incidence of lung diseases such as lung cancer and chronic obstructive pulmonary disease (COPD). These conditions often necessitate lobectomy. Moreover, advancements in surgical methods and the emergence of enhanced safer surgical tools have rendered lobectomy a more feasible and favored choice for thoracic surgeries. Notably, the lobectomy segment is currently the most rapidly expanding sector in the market. This expeditious growth can be attributed to the mounting adoption of these procedures, their abbreviated hospital stays reduced postoperative discomfort, and swifter recuperation periods compared to conventional open surgery.
The wedge resection segment is projected to grow at a CAGR of 4.8% from 2024 to 2030. Wedge resection is particularly favored for addressing early-stage lung cancer and for patients unsuitable for more extensive surgery. The anticipated expansion in this field can be ascribed to the rise in early-stage lung cancer cases, advancements in imaging technologies enabling early detection, and the increasing preference for minimally invasive approaches. Furthermore, ongoing research and development endeavors to enhance the effectiveness and safety of wedge resection procedures are also poised to fuel this growth.
Regional Insights
North America captured nearly 50% of the global market in 2023 due to several key factors. These include advancements in medical technology, leading to the development of innovative products that address patient needs. Additionally, the availability of a skilled surgical workforce, the continual introduction of new medical devices, and well-established regulatory and compensation structures have all contributed to the market's growth in this region.
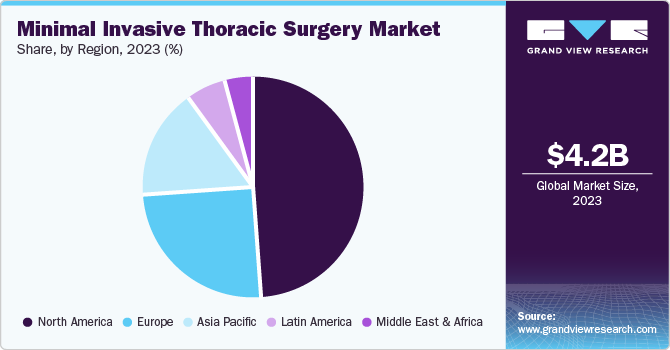
U.S. Minimal Invasive Thoracic Surgery Market Trends
The U.S. Minimally Invasive Thoracic Surgery market is expected to grow significantly from 2024 to 2030. This expansion is fueled by advancements in surgical techniques, the rising prevalence of thoracic diseases, and the increasing patient preference for less invasive treatment options.
Europe Minimally Invasive Thoracic Surgery Market Trends
Europe's minimally invasive thoracic surgery market is on the rise, driven by factors such as an aging population with chronic diseases and a growing focus on improving patient outcomes. This translates to a demand for advanced surgical instruments and techniques such as uniportal VATS. The rise of robotics and 3D visualization is also expected to influence the market significantly in the coming years.
Asia Pacific Minimally Invasive Thoracic Surgery Market Trends
The APAC minimally invasive thoracic surgery market is experiencing significant growth, driven by factors such as a rising patient population with chronic diseases and a growing awareness of the benefits of minimally invasive procedures. This translates to a higher demand for advanced surgical instruments and robotics. Countries including China and India, with their large populations, are at the forefront of this growth. This trend is expected to continue in the coming years, making the APAC region a lucrative market for minimally invasive thoracic surgery solutions.
Key Minimally Invasive Thoracic Surgery Company Insights
One of the major factors driving the market is the continuous R&D by the industry players. These initiatives by the major companies are helping improve the technology's penetration in the surgical market. Some prominent players in the global minimally invasive thoracic surgery market include:
Key Minimally Invasive Thoracic Surgery Companies:
The following are the leading companies in the minimally invasive thoracic surgery market. These companies collectively hold the largest market share and dictate industry trends.
- Medtronic
- Dextera Surgical Inc.
- Intuitive surgical Operations, Inc.
- Richard Wolf GmBH
- Grena Ltd.
- Medela
- LivaNova PLC
- Teleflex Incorporated
- Sklar Surgical Instruments.
Recent Developments
-
In November 2023, Johnson & Johnson MedTech's MONARCH Platform and MONARCH Bronchoscope achieved regulatory clearance in China, signifying a landmark advancement in minimally invasive surgical technology. This approval establishes the MONARCH system as the first robotic-assisted platform for peripheral lung procedures in the Chinese market.
-
In October 2023, The FDA approved Medtronic plc's Aurora EV-ICD systems for treating dangerously fast heart rhythms that can lead to sudden cardiac arrest (SCA). These systems are similar in size to traditional ICDs but are implanted below the left armpit with the lead placed under the breastbone using a minimally invasive approach.
Minimally Invasive Thoracic Surgery Market Report Scope
|
Report Attribute
|
Details
|
|
Market size value in 2024
|
USD 4.45 billion
|
|
Revenue forecast in 2030
|
USD 6.24 billion
|
|
Growth Rate
|
CAGR of 5.8% from 2024 to 2030
|
|
Base year for estimation
|
2023
|
|
Historical data
|
2018 - 2022
|
|
Forecast period
|
2024 - 2030
|
|
Quantitative units
|
Revenue in USD million/billion and CAGR from 2024 to 2030
|
|
Report coverage
|
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
|
|
Segments covered
|
Type, region
|
|
Regional scope
|
North America; Europe; Asia Pacific; Latin America; MEA
|
|
Country scope
|
U.S.; Canada; Mexico: U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; India; China; Australia; South Korea: Thailand; Brazil; Argentina; Saudi Arabia; UAE; South Africa; Kuwait
|
|
Key companies profiled
|
Medtronic; Intuitive Surgical Operations, Inc.; Richard Wolf GmbH; Grena Ltd.; Dextera Surgical Inc.; Teleflex Incorporated; Medela; LivaNova PLC; Sklar Surgical Instruments
|
|
Customization scope
|
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
|
|
Pricing and purchase options
|
Avail of customized purchase options to meet your exact research needs. Explore purchase options
|
Global Minimally Invasive Thoracic Surgery Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels, and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2020 to 2030. For the purpose of this study, Grand View Research has segmented the global minimally invasive thoracic surgery market report on the basis of type and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Lobectomy
-
Wedge Resection
-
Pneumonectomy
-
Others
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
Latin America
-
MEA
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait














