Market Size & Trends
The North America and Europe stroke diagnostics market size was valued at USD 2.18 billion in 2023 and is projected to grow at a CAGR of 7.2% from 2024 to 2030. The growing aging population, with an increased incidence of strokes among the elderly, is one of the key drivers for the market growth. This demographic trend highlights the increasing requirement for sophisticated diagnostic tools and services to address the elevated susceptibility of the aging population to health challenges related to stroke. For instance, the death rate for strokes in the U.S. increased from 38.8 per 100,000 in 2020 to 41.1 per 100,000 in 2021. Each year, more than 795,000 people in the U.S. suffer from a stroke.
It is anticipated that increased awareness regarding early chance of stroke and its causes will positively influence market development in the coming years. The implementation of smoking cessation by the U.S. government and other such initiatives are anticipated to boost demand for stroke diagnostics in the coming years.
Furthermore, both regions are experiencing an increasing number of elderly individuals, who are more susceptible to strokes. This results in an increase in the demand for diagnostic tools and services that enable the prompt and efficient identification of strokes. There is an increasing prevalence of stroke risk factors, such as high blood pressure, diabetes, obesity and changing lifestyle and habits. For instance, a new study reveals that current smokers are at a higher risk of getting a stroke, while smoking for more than 10 hours a week nearly doubles the stroke risk.
Product Insights
Computed Tomography Scan (CT scan) dominated the market and accounted for a share of 52.5% in 2023. This is owing to the availability of noninvasive diagnostic techniques and accurate results. Spiral CT provides thorough evaluation of body parts along with significant improvement in multiplanar and 3D renderings. CT scans are very efficient at detecting brain hemorrhages, which are the main feature of hemorrhagic strokes.
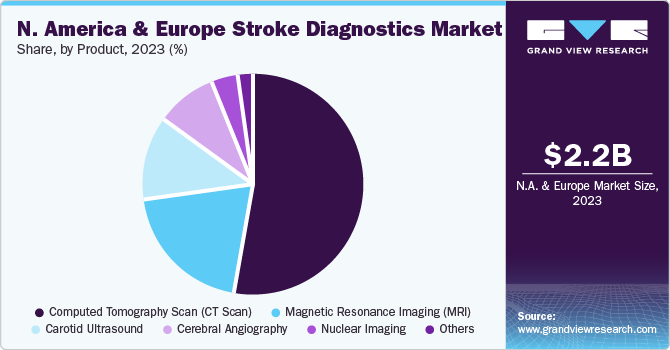
Carotid ultrasound (CUS) is expected to register the fastest CAGR of 9.6% during the forecast period. CUS uses sound waves to show the carotid arteries. It's painless and comfortable for patients. No needles or radiation are used to check the carotid arteries, which supply blood to the brain. These arteries are a big risk factor for stroke. CUS can be done in outpatient clinics or doctors’ offices. This makes it easier for patients and reduces wait times. Also, new technology has made it easier to make portable ultrasound systems. This allows CUS to be used in remote locations or for mobile stroke assessment units.
Regional Insights
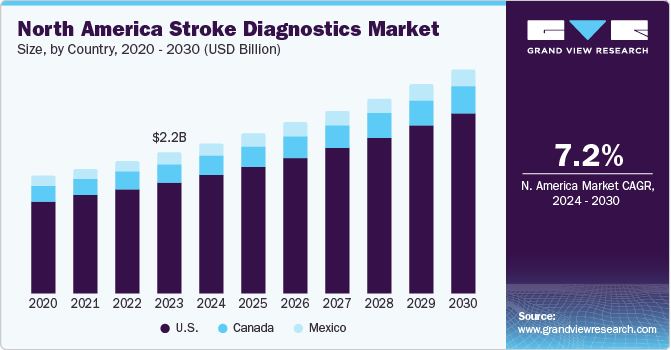
North America stroke diagnosis market dominated the market in 2023 with share of 59.6%. The National Stroke Association highlights that stroke is the fifth-leading cause of death in the U.S. and is a large factor for adults’ disability. As per The World Health Organization, diabetes can lead to stroke. Therefore, with the increasing prevalence rate of diabetes, the chances of stroke are anticipated to increase.
U.S. Stroke Diagnostics Market Trends
The stroke diagnostics market in the U.S. dominated the market with a share of 79.0% in 2023 as a result of all factors like growing aging population and with an increased incidence of strokes among the elderly. AI-powered technologies are advancing the diagnosis of strokes by increasing precision, cutting down on interpretation times, and possibly allowing for earlier intervention .Faster identification in emergency situations and possibly even at home is made possible by advancements in CT scanners and possibly even portable stroke diagnostic tools. Campaigns for education and public health are raising awareness of the signs of a stroke and the significance of getting medical help right away. The cost of stroke diagnostic procedures is rising, making them more affordable for patients through the use of private insurance companies and government healthcare programs like Medicare and Medicaid.
Europe Stroke Diagnostics Market Trends
Europe Stroke Diagnostics market was identified as a lucrative region in 2023. The increasing emphasis on prompt and precise stroke diagnosis is propelling the market growth. Europe has an aging population. Age is a significant risk factor for stroke, which is expected to increase the demand for stroke diagnostic services. Chronic conditions such as high blood pressure, diabetes, and obesity are becoming increasingly common in Europe. This increase in risk factors results in a higher incidence of strokes, further fueling the need for efficient diagnostic tools.
The UK stroke diagnostics market is expected to grow rapidly in the coming years. European nations such as the UK is experiencing an increasing number of elderly individuals. This age group is more susceptible to stroke, which is increasing the demand for stroke diagnostic services.
Asia Pacific Stroke Diagnostics Market Trends
Asia Pacific stroke diagnostics market is anticipated to witness significant growth in coming years. The growth is fueled by a substantial and expanding population that is susceptible to stroke and an increasing expenditure on healthcare. The emerging economies in APAC are experiencing an increase in private healthcare expenditure and an expanding middle class sector. Countries such as Japan and South Korea have established healthcare systems with widespread adoption of advanced diagnostic technologies.
Key North America And Europe Stroke Diagnostics Company Insights
Some of the key companies in the stroke diagnostic market include Siemens Healthcare, Boston Scientific Corp., GE Healthcare, Philips Healthcare, and Neusoft Medical Systems. Organizations are concentrating on expanding their customer base to achieve a competitive advantage in the market. Hence, major stakeholders are implementing various strategic actions, including mergers, acquisitions, and collaborations with other leading firms.
-
Siemens Healthcare provides imaging technologies like CT and MRI systems. In addition, Siemens offers extensive IT solutions for managing and analyzing images, which help streamline stroke care processes and enhance patient results.
Key North America Stroke Diagnostics Stroke Diagnostics Companies:
- Siemens Healthineers AG
- Cordis.
- Boston Scientific Corporation
- GE HealthCare
- Koninklijke Philips N.V.
- TOSHIBA CORPORATION
- Hitachi High-Tech Corporation
- Neusoft Corporation
Recent Developments
-
In February 2024, Upfront Diagnostics announced to launch LVOne, lateral flow test, that is anticipated to enable paramedics to test patients suspected to have had a stroke of large vessel occlusions (LVO), triage patients and to offer best possible care
-
In December 2022, Fujifilm India and Soorya Diagnostics LLP partnered to install Computed Tomography Scan in Tirur. It comes with AI technique, that improves low radiation dose CT images quality and reduced side effects of increased quantum mottle image noise.
North America And Europe Stroke Diagnostics Market Report Scope
|
Report Attribute
|
Details
|
|
Market size value in 2024
|
USD 2.33 billion
|
|
Revenue forecast in 2030
|
USD 3.53 billion
|
|
Growth rate
|
CAGR of 7.2% from 2024 to 2030
|
|
Base year for estimation
|
2023
|
|
Historical data
|
2018 - 2022
|
|
Forecast period
|
2024 - 2030
|
|
Quantitative units
|
Revenue in USD billion and CAGR from 2024 to 2030
|
|
Report coverage
|
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
|
|
Segments covered
|
Product, region
|
|
Country scope
|
U.S.; Canada; Mexico; Germany; UK; France
|
|
Key companies profiled
|
Siemens Healthineers AG; Cordis.; Boston Scientific Corporation; GE HealthCare; Koninklijke Philips N.V.; TOSHIBA CORPORATION; Hitachi High-Tech Corporation; Neusoft Corporation
|
|
Customization scope
|
Free report customization (equivalent up to 8 analysts' working days) with purchase. Addition or alteration to country, regional & segment scope.
|
|
Pricing and purchase options
|
Avail customized purchase options to meet your exact research needs. Explore purchase options
|
North America And Europe Stroke Diagnostics Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the North America and Europe stroke diagnostics market report based on product and region.
-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway













