- Home
- »
- Clinical Diagnostics
- »
-
North America In Vitro Diagnostics Market Share Report, 2030GVR Report cover
![North America In Vitro Diagnostics Market Size, Share & Trends Report]()
North America In Vitro Diagnostics Market Size, Share & Trends Analysis Report By Product (Instruments, Reagents, Services), By Technology, By Application, By End-use, By Test Location, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-028-3
- Number of Report Pages: 280
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Report Overview
The North America in vitro diagnostics market size was valued at USD 50.16 billion in 2022 and is expected to decline at a compound annual growth rate (CAGR) of 2.89% from 2023 to 2030. The decline in demand for COVID-19 infection detection tests is projected to hamper the growth over the forecast period. Whereas, excluding COVID-19, the market is set to gain momentum in coming years owing to the increasing prevalence of target diseases and the introduction of cutting-edge technologies that provide novel ways to detect infections and other diseases. For instance, in December 2022, AXIM Biotechnologies Inc. developed a novel dual IgE/MMP-9 rapid ophthalmological diagnostic test, which is capable of diagnosing IgE and MMP-9 in a single test.
Increasing incidences of neurological, cardiovascular, and genetic disorders are projected to accelerate North America in vitro diagnostics industry's growth. NGS-based genetic prenatal tests are becoming more common and their adoption is rising due to the high accuracy of these tests and increasing awareness among consumers.
Key players such as Illumina, Inc., Natera, Inc., Eurofins, and F. Hoffmann-La Roche Ltd. offer prenatal screening tests that allow the detection of common chromosomal abnormalities such as Down’s syndrome. In addition, the recent market introduction of accurate and precise diagnostic solutions is also fueling the North America in vitro diagnostics industry. For instance, in April 2021, Siemens Healthineers received a CE mark for its Atellica VTLi Patient-Side Immunoassay Analyzer.
Improvements in technology and the consequent introduction of cost-effective & high-quality medical solutions aimed at achieving lab automation are acting as growth drivers for the North America in vitro diagnostics industry. For instance, in July 2022, BioGX, a leader in molecular diagnostics, launched POC CE-marked COVID-19 tests on its pixl platform. Similarly, in October 2021, Bio-Rad Laboratories, Inc. launched CFX Opus 384 Dx System and CFX Opus 96 Dx System, a qPCR detection system. This recently launched system provides precise and accurate quantification to improve workflow efficiencies for diagnostic testing.
Moreover, growing efforts from government and non-government organizations to promote the development of novel diagnostic solutions are expected to offer lucrative growth opportunities for the North America in vitro diagnostics industry. For instance, in October 2022, Day Zero Diagnostics Inc. announced that it had received additional funding of USD 8.2 million from CARB-X for the development of accurate and cutting-edge diagnostic solutions for infectious diseases. Similarly, in October 2021, the NIH granted funding of USD 78 million to develop rapid diagnostic solutions for COVID-19. This funding aimed to develop PoC solutions for detecting multiple respiratory infections.
Furthermore, the favorable regulatory framework for in vitro diagnostic devices and market players’ efforts to develop novel IVD products are anticipated to support market expansion throughout the forecast period. For instance, in January 2021, Abbott received the U.S. FDA approval for its rapid handheld Traumatic Brain Injury (TBI) blood test. This first-of-its-kind test is intended for assessing mild TBIs and concussions in patients. Moreover, in May 2022, the U.S. FDA granted marketing approval for a new IVD test for the early detection of amyloid plaques associated with Alzheimer’s disease.
Product Insights
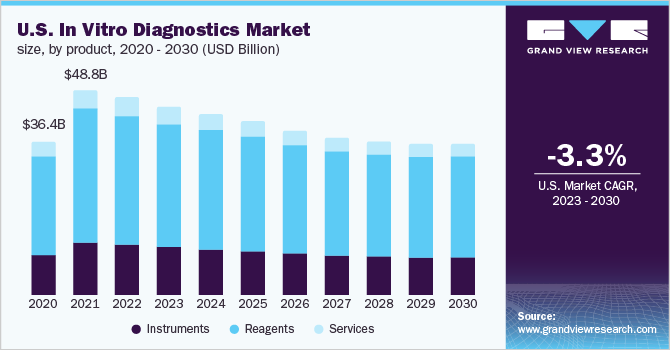
The reagents segment held the largest share of 65.5% in 2022 and is anticipated to advance at a steady CAGR during the projected period. Factors such as extensive R&D initiatives being undertaken by major market players for the development of novel tests for different applications and the commercialization of new reagents are likely to drive market growth.
For instance, in March 2021, Promega Corporation introduced XpressAmp direct amplification reagents, which facilitate automation-friendly RNA extraction-free sample preparation. Moreover, in November 2021, 28 Fungal Analyte Specific Reagents (ASRs) were launched by Applied BioCode to sell to clinical laboratories and IVD manufacturers.
The instruments segment held the second largest revenue share in 2022; the high revenue share of the segment can be attributed to the rising demand for PoC testing instruments, technological advancements, and the rising demand for advanced instruments by end-users. Furthermore, key manufacturers are focused on developing new technologies with higher efficiency & accuracy. For instance, the VIDAS and MINI VIDAS systems by bioMérieux are multiparametric immunoassay systems designed to offer highly accurate & precise laboratory results.
Technology Insights
The molecular diagnostics segment dominated the market for North America in vitro diagnostics owing to factors such as rapid evolution in technology, increasing burden of target diseases, the outbreak of COVID-19, and surge in product launches by leading participants. For instance, in November 2022, Roche planned to launch the cobas 5800 system, a compact and fully automated lab instrument that offers flexible PCR testing solutions, in the U.S.
Moreover, the presence of flexible regulations for manufacturers to combat the rising number of infectious diseases is anticipated to accelerate North America in vitro diagnostics industry growth. For instance, in March 2021, the U.S. FDA issued a EUA for Abbott’s molecular test to detect COVID-19. Moreover, the launch of initiatives & programs is further propelling the demand for molecular diagnostics to reduce disease burden. For instance, in March 2022, Molecular Characterization Initiative was launched by the National Cancer Institute to provide childhood cancer molecular characterization.
The rising demand for regular health checkups, the surge in the prevalence of hematological disorders, and the rising demand for POC & portable devices are some factors responsible for the growth of the hematology segment. The rising demand for benchtop and portable devices is pushing manufacturers to develop hematology instruments with new features and enhanced performance. For instance, in July 2022, HORIBA Medical launched Yumizen 550 and 500 in its hematology product portfolio. Similarly, in August 2020, IDEXX Laboratories, Inc. launched the ProCyte One Hematology Analyzer, which delivers accurate and consistent results with operability in PoC settings.
Application Insights
In 2022, the infectious disease segment held the largest market share of 60.4% owing to the rising burden of infectious diseases in the region and the increasing uptake of IVD products for the detection of infectious diseases. The COVID-19 pandemic has increased segment growth exponentially. In addition, leading participants are engaged in commercializing precise testing solutions for infectious diseases. For instance, in October 2021, QIAGEN launched the QuantiFERON-TB test for tuberculosis infection. The newly launched product aimed to reduce the rising tuberculosis burden in the region.
The diabetes segment held the second largest share due to the growing trend of a sedentary lifestyle in North America, the prevalence of obesity, and the rising demand for homecare diagnostic solutions. Several companies are introducing portable and home-use devices owing to their high demand. For instance, in May 2022, LabCorp launched the LabCorp OnDemand at-home collection kit, which can measure HbA1c from small blood samples.
The oncology segment is anticipated to exhibit a lucrative growth rate throughout the forecast period. The increasing burden of cancer in the U.S. & Canada, rising demand for advanced screening solutions, and increasing awareness for early disease diagnosis among people are all expected to support segment expansion.
Test Location Insights
Most tests are performed in central laboratory settings. However, the increased focus of the regulatory bodies on self-test or OTC molecular diagnostics to lower the burden on laboratories is boosting the OTC test market’s growth. For instance, in March 2021, Cue Health, Inc. announced the U.S. FDA approval of its OTC and at-home self-test for SARS-CoV-2, making it the first non-prescription diagnostic test in the U.S. Moreover, in May 2021, the retail chain Kroger Co. announced the availability of self-testing kits by Abbott across its store to increase overall access to test kits.
The point-of-care segment is projected to register a robust growth rate throughout the projected period. The rising developments of tests that provide faster detection are projected to boost the segment’s growth. These POC tests have revolutionized the market by decreasing the processing time and augmenting rapid decision-making. For instance, in April 2021, the U.S. FDA approved amended EUA requests for various tests, expanding POC and OTC testing options for COVID-19.
End-use Insights
The hospitals segment captured the largest revenue share in 2022. Factors such as the rising demand for affordable services, increasing hospitalization rates, the surging trend of personalized treatments, and the presence of favorable reimbursement policies are fueling the segment’s expansion. Moreover, the increasing incidence of hospital-acquired infections in hospitalized patients is expected to support market growth. According to the CDC, around 5% of hospitalized patients suffer from MRSA infections and carry the bacteria.
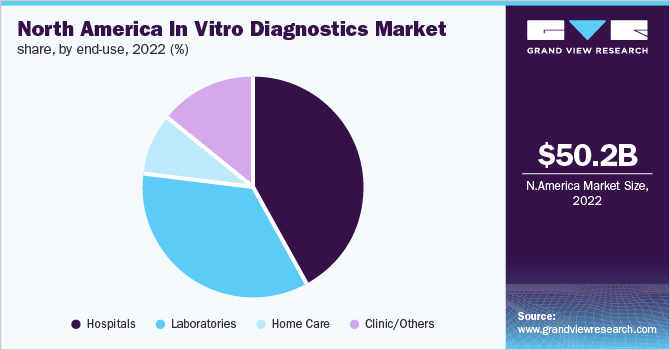
On the other hand, home care is likely to show a positive growth rate during the coming years. The rising demand for homecare in vitro diagnostic devices, the surge in the geriatric population, and the robust availability of portable home-use IVD products are facilitating segment expansion. Moreover, favorable government initiatives to promote home care diagnosis are fueling segment growth. For instance, the U.S. FDA has authorized a large number of over-the-counter COVID-19 diagnostic tests for home use.
Regional Insights
The U.S. led the North America in vitro diagnostics industry in 2022 due to the presence of many strong players coupled with various strategic initiatives undertaken by them. Moreover, the high burden of target diseases, rising demand for advanced diagnostic solutions at different healthcare facilities, and surge in demand for POC tests are also anticipated to support the growth.
Canada is projected to witness the fastest growth rate over the forecast period. The increasing geriatric population, large patient pool with the increasing prevalence of chronic diseases, and supportive reimbursement policies in the country are the factors bolstering the country’s market growth. Moreover, the introduction of technologically advanced products is likely to cater to the nation's market. For instance, in October 2021, Bio-Rad introduced the CFX Opus 384 Dx System and CFX Opus 96 Dx Systems, which are real-time PCR detection systems.
Key Companies & Market Share Insights
Key players are adopting strategies such as new product developments, mergers & acquisitions, and partnerships to increase their market share. Market players such as Abbott, bioMérieux SA, F. Hoffmann-La Roche AG, and others are actively involved in the development of novel and precise IVD products. For instance, in November 2022, Sense Biodetection announced a strategic agreement with Bio Nuclear Diagnostics, Inc. for the distribution of the Veros point-of-care molecular testing platform in Canada. Some prominent players in the North America in vitro diagnostics market include:
-
Abbott
-
bioMérieux SA
-
Bio-Rad Laboratories, Inc.
-
BD
-
Siemens Healthcare GmbH
-
QIAGEN
-
Quidel Corporation
-
F. Hoffmann-La Roche Ltd
-
Sysmex Corporation
-
Charles River Laboratories International, Inc.
-
Quest Diagnostics
-
Agilent Technologies, Inc.
-
Danaher
North America In Vitro Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 48.19 billion
Revenue forecast in 2030
USD 39.67 billion
Growth rate
CAGR of -2.89% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Volume in number of tests & instruments, revenue in USD million, and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, technology, application, end-use, test location, region
Regional scope
North America
Country scope
U.S.; Canada
Key companies profiled
Abbott; bioMérieux SA; Bio-Rad Laboratories, Inc.; BD; Siemens Healthcare GmbH; QIAGEN; Quidel Corporation; F. Hoffmann-La Roche Ltd; Sysmex Corporation; Charles Rivers Laboratories International, Inc.; Quest Diagnostics; Danaher
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
North America In Vitro Diagnostics Market Segmentation
This report forecasts the volume and revenue growth at the regional and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the North America in vitro diagnostics market report based on product, technology, application, end-use, test location, and country.
-
Product Outlook (Volume, Number of Tests and Instruments; Revenue, USD Million, 2018 - 2030)
-
Instruments
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Reagents
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Services
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
-
Technology Outlook (Volume, Number of Tests and Instruments; Revenue, USD Million, 2018 - 2030)
-
Immunoassay
-
Product
-
Instruments
-
Reagents
-
Services
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Hematology
-
Product
- Instruments
- Reagents
- Services
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Clinical Chemistry
-
Product
-
Instruments
-
Reagents
-
Services
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Molecular Diagnostics
-
Product
-
Instruments
-
Reagents
-
Services
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Coagulation
-
Product
- Instruments
- Reagents
- Services
-
End-Use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Microbiology
-
Product
-
Instruments
-
Reagents
-
Services
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Others
-
Product
-
Instruments
-
Reagents
-
Services
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
-
Application Outlook (Volume, Number of Tests and Instruments; Revenue, USD Million, 2018 - 2030)
-
Infectious Disease
-
Disease
-
Methicillin-resistant Staphylococcus Aureus (MRSA)
-
Clostridium Difficile
-
Vancomycin-resistant Enterococci (VRE)
-
Carbapenem-resistant Bacteria
-
Flu
-
Respiratory Syncytial Virus (RSV)
-
Candida
-
Tuberculosis and Drug-resistant TBA
-
Meningitis
-
Gastrointestinal Panel Testing
-
Chlamydia
-
Gonorrhea
-
HIV
-
Hepatitis C
-
Hepatitis B
-
Other Infectious Disease
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Diabetes
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Oncology
-
Disease
-
Breast Cancer
-
Prostate Cancer
-
Colorectal Cancer
-
Cervical Cancer
-
Kidney Cancer
-
Liver Cancer
-
Blood Cancer
-
Lung Cancer
-
Other Cancer
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Cardiology
-
Disease
-
Coronary Heart Disease
-
Stroke
-
Peripheral Arterial Disease
-
Aortic Disease
-
Others
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Nephrology
-
Disease
-
UTI
-
Kidney Disease
-
Diabetes
-
Others
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Autoimmune Diseases
-
Disease
-
Rheumatoid Arthritis
-
Inflammatory Bowel Disease
-
Osteoporosis
-
Celiac Disease
-
Fibromyalgia
-
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Drug Testing
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
Others
-
End-use
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
-
-
End-use Outlook (Volume, Number of Tests and Instruments; Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Laboratories
-
Home Care
-
Clinic/Others
-
Physician Office
-
Pharmacy & Retail Clinics
-
Non-practice Clinics
-
Urgent Care Clinics
-
Others
-
-
-
Test Location Outlook (Revenue, USD Million, 2018 - 2030)
-
Point-of-Care
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Frequently Asked Questions About This Report
b. The North America in vitro diagnostics market size was estimated at USD 50.16 billion in 2022 and is expected to reach USD 48.19 billion in 2023.
b. The North America in vitro diagnostics market is expected to grow at a compound annual growth rate of -2.89% from 2023 to 2030 and is expected to reach USD 39.67 billion by 2030.
b. The molecular diagnostics segment is expected to dominate the North America in vitro diagnostics market with a share of 38.50% in 2022 due to the rapid evolution in technology, increasing burden of targeted diseases, and increase in the introduction of novel products in the region.
b. Some key players operating in the North America in vitro diagnostics market include Abbott, F. Hoffmann-La Roche Ltd, bioMérieux SA, Bio-Rad Laboratories, Inc., BD, Siemens Healthcare GmbH, QIAGEN, and Quidel Corporation among others.
b. The increasing prevalence of infectious & chronic diseases, introduction of novel diagnostic products, favorable government initiatives, and presence of strong market players in the region are the major factors driving the North America in vitro diagnostics market growth over the forecast period.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





