- Home
- »
- Medical Devices
- »
-
Peripheral Vascular Devices Market Size & Share Report, 2030GVR Report cover
![Peripheral Vascular Devices Market Size, Share & Trends Report]()
Peripheral Vascular Devices Market Size, Share & Trends Analysis Report By Type (Peripheral Stents, PTA Balloons, Catheters, Endovascular Aneurysm Repair Stent Grafts, Plaque Modification Devices), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: 978-1-68038-237-2
- Number of Pages: 190
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry: Healthcare
Report Overview
The global peripheral vascular devices market size was valued at USD 6,500 million in 2022 and is expected to exhibit a compound annual growth rate (CAGR) of 7.4% from 2023 to 2030. This growth can be attributed to increase in the prevalence of peripheral vascular diseases (PVDs). According to Center for Disease Control and Prevention (CDC), in North America approximately 6.5 million people aged 40 years and over live with the peripheral arterial disease (PAD). Smoking, atherosclerosis, diabetes, high cholesterol, and high blood pressure are some of the key high-risk factors that contribute to rise in new cases of PAD worldwide. The peripheral stents, catheters, and Percutaneous Transluminal Angioplasty (PTA) Balloons are some of key devices used by surgeons to avoid artery blockage and smoothen blood circulation in patients with PAD. Thus, increasing prevalence of PVD is anticipated to propel demand for these devices in the coming years, thereby, driving the market growth.
Furthermore, several key players are undertaking various initiatives such as merger & acquisition and new product launch to expand business portfolio. For instance, in September 2021, Abbott acquired Walk Vascular, LLC, a medical device company with a portfolio of a minimally invasive mechanical aspiration thrombectomy system which includes next generation JETi AIO Peripheral Thrombectomy System and JETi Peripheral Thrombectomy System to remove peripheral blood clots. This acquisition strengthens Abbott’s vascular business and is expected to drive market growth.
The market for is seeing an increase in the use of bio-absorbable stents to reduce thrombogenic risk factors and improve clinical applicability. The risk of late stent thrombosis is eliminated with bio-absorbable stents, which are constructed of naturally soluble substances that dissolve after implantation. They aid in defending the body against swelling or decreased blood flow, brought on by late-stent thrombosis. Thrombosis is the condition where the stent used for treatment stays inside the body for a prolonged period.
The key players are concentrating more on manufacturing and selling bio-absorbable stents. For instance, in January 2021, the SYNERGY Megatron bio absorbable polymer coronary stent system, launched by Boston Scientific, received the U.S. FDA approval. The stent is used to treat coronary artery disease by inserting the stents delivery balloon catheter into a blood vessel in the patient’s arm or groin. It is designed for large proximal vessels, such as lesions on the ossicles, and bifurcations. The diameters range from 3.5 mm to 5.0 mm. It has increased strength and the ability to shape vessels into tapered shapes. It is constructed of a unique platinum chromium alloy that is visible on angiography and can aid in precise stent placement.
The outbreak of COVID-19 had a significant impact on the peripheral vascular devices market. The viral infection and strict measures undertaken by the government to curb the spread of the infection led to the decrease in the hospital admissions related to coronary heart related disease conditions. According to European Heart Journal Study, in October 2021, it showed that the COVID-19 outbreak was associated with reduction of new hospital admission for ACacute coronary syndromes (ACS), primary coronary angioplasty (PPCI), and ST Elevation Myocardial Infarction (STEMI) worldwide by 27.5%, 26.7%, and 20%, respectively. Therefore, low demand was seen for peripheral vascular devices during the pandemic. However, post COVID-19 pandemic, the demand for PVD again started increasing due to relaxation in lockdowns and increasing hospitalization for heart conditions globally.
However, the presence of highly effective alternative treatment options and its growing use is expected to restrain market growth over the forecast period. Anticoagulant drug is considered as a first line treatment option for patients with PVDs. The high efficacy and tolerability associated with anticoagulant, such as Dabigatran, is resulting in their wide use and creating major hurdle for growing use of peripheral vascular devices. Other medications such as vasodilators, cholesterol-lowering drugs, and antiplatelet medicines are also used as an alternative to the PADs.
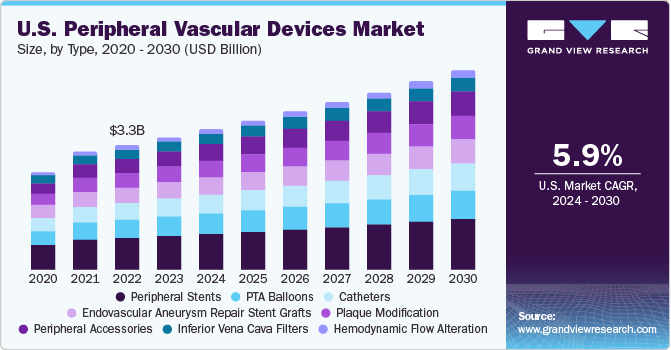
Type Insights
By type, the peripheral stents segment held largest share of 25.7% in 2022. This dominance can be attributed to the increasing use of stents owing to high efficacy, safety, and tolerability associated with it. According to the U.S. Food and Drug Administration (FDA), Paclitaxel coated balloon and stents are recommended to treat patients with new and recurring atherosclerotic lesion in the femoropopliteal artery condition. It works to mechanically open the blocked blood vessel. Some of the FDA approved peripheral stents are Zilver PTX and ELUVIA drug-eluting vascular stent system manufactured by Cook Ireland Ltd and Boston Scientific Corporation. The introduction of new peripheral stent is expected to drive market growth.
Furthermore, the peripheral accessories segment is anticipated to grow at a fastest rate over the forecast period. The development of improved peripheral accessories that are helpful during peripheral vascular procedures and improve accessibility is likely to increase their use in the upcoming years.
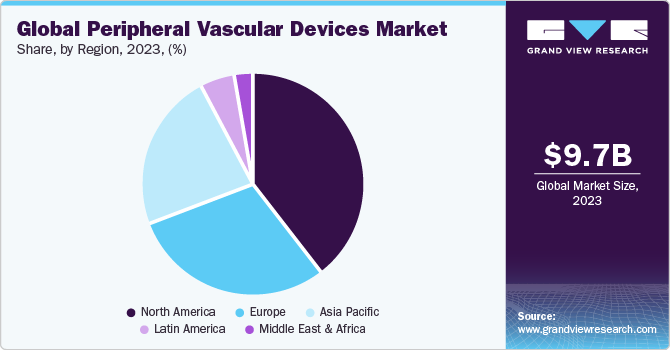
Regional Insights
In 2022, North America dominated the peripheral vascular devices market with a share of 39.7%. This is due to the factors such as increasing incidence of cardiovascular diseases, increasing geriatric population which drives the market. Also, due to the increasing number of cases with cardiovascular diseases in the U.S., the market growth is rising. Moreover, North America has presence of majority of the industry key players. Many of the industry players have enhanced their product portfolio by entering into strategic collaborations, mergers, partnerships and expanding their novel product launch which boosts the peripheral vascular devices market.
For instance, in December 2021 , Phillips, a world leader in technology entered into an agreement to acquire Vesper Medical Inc. The latter company is a U.S.-based medical technology company that manufactures minimally invasive peripheral vascular devices. Vesper Medical will add an advanced portfolio of venous stent for the treatment of deep venous illness to Philips' line of diagnostic and therapeutic devices. Such advancements spur the market growth, creating lucrative opportunities for market growth.
Asia Pacific is anticipated to witness fastest growth over the forecast period owing to the rising incidence of diabetes which leads to the increasing incidence of peripheral vascular disorders. Moreover, growing demand for minimal invasive treatments, technologically advanced treatments, improving healthcare infrastructure are some of the factors projected to lead the market in the upcoming years.
Key Companies & Market Share Insights
Various strategies such as product development, partnerships, funding’s, investments, etc. adopted by key players is driving the market. For instance, in August 2022 , the privately held company, Obsidio, Inc. was acquired by Boston Scientific Corporation. Obsidio created the FDA approved Gel Embolic Material (GEM) technology, which is utilized to embolize blood vessels in the peripheral vasculature. The acquisition will strengthen the company’s interventional oncology portfolio and embolization sector with addition of the GEM technology. Some prominent players in the global peripheral vascular devices market include:
-
Abbott Laboratories
-
Angioscore Inc.
-
Edward Lifesciences Corporation
-
Medtronic Inc.
-
St. Jude Medical
-
Teleflex Medical
-
Volcano Corporation
-
Boston Scientific Corporation
-
Teleflex Medical
-
Cook Group Inc.
-
Cordis Corporation
-
Covidien
-
W.L.Gore and Associates Ltd.
-
Angioscore Ltd.
Peripheral Vascular Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 7,000 million
Revenue forecast in 2030
USD 11,574.4 million
Growth rate
CAGR of 7.4% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Denmark; Norway; Sweden; China; Japan; India; South Korea; Australia; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Abbott Laboratories; Angioscore Inc.; Edward Lifesciences Corporation; Medtronic Inc.; St. Jude Medical; Teleflex Medical; Volcano Corporation; Boston Scientific Corporation; Teleflex Medical; Cook Group Inc.; Cordis Corporation; Covidien; W.L.Gore and Associates Ltd.; and Angioscore Ltd.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
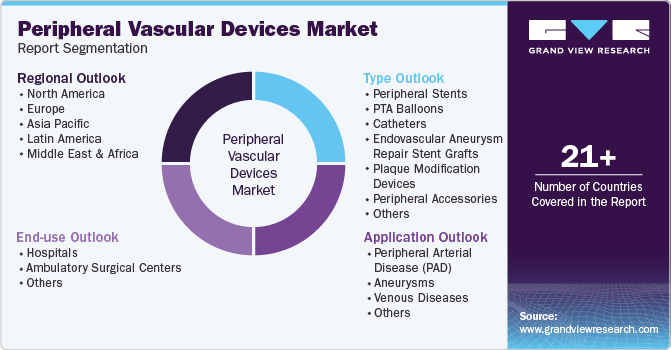
Global Peripheral Vascular Devices Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global peripheral vascular devices market report based on type, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Peripheral Stents
-
Iliac Artery Stents
-
Femoral Artery Stents
-
Carotid Artery Stents
-
Renal Artery Stents
-
Other Peripheral Stents
-
-
PTA Balloons
-
Catheters
-
Angiography Catheters
-
Guiding Catheters
-
IVUS/OCT Catheters
-
-
Endovascular Aneurysm Repair Stent Grafts
-
Thoracic Endovascular Aneurysm Stent Grafts
-
Abdominal Endovascular Aneurysm Stent Grafts
-
-
Plaque Modification Devices
-
Atherectomy Devices
-
Thrombectomy Devices
-
-
Peripheral Accessories
-
Guidewires
-
Workhorse Guidewires
-
Specialty Guidewires
-
Extra Support Guidewires
-
Frontline Finesse Guidewires
-
-
Peripheral Vascular Closure Devices
-
Balloon Inflation Devices
-
Introducer Sheaths
-
-
Inferior Vena Cava Filters
-
Permanent Filters
-
Retrievable Filters
-
-
Hemodynamic Flow Alteration Devices
-
Chronic Total Occlusion Devices
-
Embolic Protection Devices
-
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
MEA
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global peripheral vascular devices market size was estimated at USD 6,500 million in 2022 and is expected to reach USD 7,000 million in 2023.
b. The global peripheral vascular devices market is expected to grow at a compound annual growth rate of 7.4% from 2023 to 2030 to reach USD 11,574.4 million by 2030.
b. Peripheral Vascular Stents dominated the type segment held largest share of 25.7% in 2022. This dominance can be attributed to the increasing use of stents owing to high efficacy, safety, and tolerability associated with it. According to the U.S. Food and Drug Administration (FDA), Paclitaxel coated balloon and stents are recommended to treat patients with new and recurring atherosclerotic lesion in the femoropopliteal artery condition.
b. Some key players operating in the peripheral vascular devices market include Abbott Laboratories, Angioscore Inc., Edward Lifesciences Corporation, Medtronic Inc., St. Jude Medical, Teleflex Medical, Volcano Corporation, Boston Scientific Corporation, Teleflex Medical, Cook Group Inc., Cordis Corporation, Covidien, W.L.Gore and Associates Ltd., and Angioscore Ltd.
b. Key factors that are driving the market growth include increasing incidence of cardiac diseases, rising global geriatric population base triggering incidence rates of target diseases; novel product launch; increase in collaboration, mergers by the industry key players.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





