- Home
- »
- Biotechnology
- »
-
Protein Binding Assays Market Size & Share Report, 2030GVR Report cover
![Protein Binding Assays Market Size, Share & Trends Report]()
Protein Binding Assays Market (2024 - 2030) Size, Share & Trends Analysis Report By Technology (Ultrafiltration, Ultracentrifugation), By Product & Services (Instrument), By End Use (CROs), By Region, And Segment Forecasts
- Report ID: GVR-3-68038-593-9
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Protein Binding Assays Market Summary
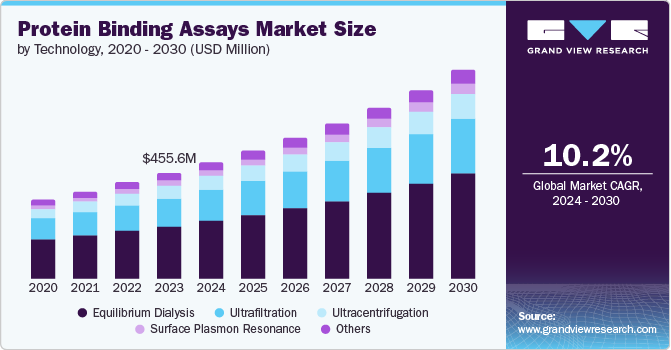
The global protein binding assays market size was estimated at USD 455.6 million in 2023 and is projected to reach USD 896.3 million by 2030, growing at a CAGR of 10.2% from 2024 to 2030. Protein binding assays from biochemistry and molecular biology are assessment methods to study the molecular behavior between proteins, nucleic acid, or a combination of proteins and nucleic acid.
Key Market Trends & Insights
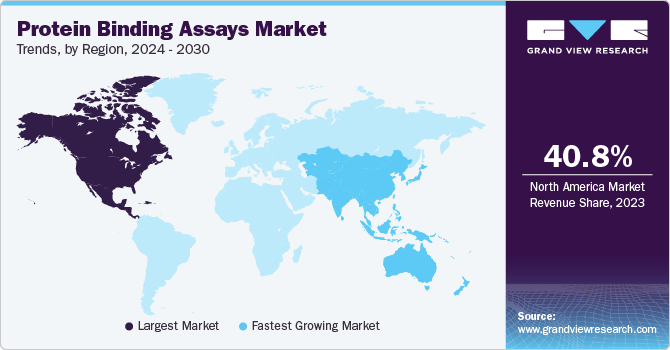
- The North America protein binding assays dominated the global market with a share of 40.8% in 2023.
- Asia Pacific protein binding assays market is expected to be the fastest-growing with a CAGR of 11.1% over the forecast period.
- Based on product & services, the services segment dominated with a market share of 44.7% in 2023.
- Based on end-use, the pharmaceuticals and biotechnology segment dominated with a 46.4% market share in 2023.
- Based on technology, the equilibrium dialysis dominated with a share of 49.7% in 2023.
Market Size & Forecast
- 2023 Market Size: USD 455.6 Million
- 2030 Projected Market Size: USD 896.3 Million
- CAGR (2024-2030): 10.2%
- North America: Largest market in 2023
- Asia Pacific: Fastest growing market
The use of advanced technologies for preclinical assessments, and funding for novel drug discovery contribute to the capabilities of these assessments.
The increase in protein binding tests is becoming necessary due to the prevalence of chronic diseases. This drives the need for medications for better treatment in patients. Other factors such as the growth in spending on research and development, and the launch of novel drugs with high efficacy are encouraging market growth. Besides, the rise in research and development has resulted in easy and faster assay methods based on a high frequency of usage.
Protein binding assays enhance the targeted therapies and minimize the quantity of target molecules. The growing demand for protein binding assays in the past few years owing to the significance of proteins for biological processes is driving the market growth. In addition, protein binding tests produce personalized medicine and medical therapy based on individual characteristics. The technological advances lead to the production of assays with accuracy, and sensitivity, that can be incorporated into analytical tools. The protein binding assay methods have become precise, effective, and efficient in the past several years. The partnerships and collaborations between pharmaceutical industries, institutions, and contract research organizations have resulted in the emergence of cutting-edge technologies to facilitate drug discovery and development.
Product & Services Insights
The services segment dominated with a market share of 44.7% in 2023. Technological advancements, strategic investments, and increasing consumer demand constitute market growth. Additionally, the major companies in the sector are focusing on innovation to meet a wide range of needs for their customers, incorporation of advanced technology such as the Internet of Things and artificial intelligence has significantly impacted efficiency and functionality. The launching of new products with enhanced capabilities is made due to which the market is expected to grow.
The kits and reagents segment is expected to witness a fast-growing CAGR of 9.8% over the forecast period. Factors such as ready-to-use customization, and the ability to measure protein biomarkers used for discovering new drugs and disease diagnostics contribute to the segment’s growth. In addition, the increasing number of repeat purchases from laboratories is also significant to the demand.
End-use Insights
The pharmaceuticals and biotechnology segment dominated with a 46.4% market share in 2023. Factors such as a wide and strong distribution network and significant investments in drug discovery boost the segment growth. The pharmaceuticals cater to the end user’s needs and medications are offered based on the disease profile which is prescribed by a medical professional.
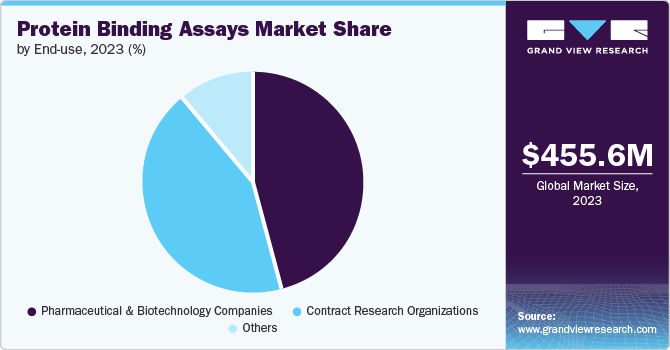
The CRO (Contract Research Organization) is expected to be the fastest-growing segment with a CAGR of 10.5% during the forecast period. Factors such as expertise in assisting biologics developers to navigate through complex processes and regulations across various geographies drive their significance. Besides, CRO support for biopharmaceutical assessment methods is crucial. In addition, major organizations are increasingly outsourcing research tasks and activities to third parties to optimize their workload. This further enhances the research quality.
Technology insights
The equilibrium dialysis dominated with a share of 49.7% in 2023. Scientists and researchers prefer this technique due to its low price and high accuracy. In addition, the increased focus on the discovery of new drugs and development in the pharmaceutical and biotechnological industry further fuels the market growth.
The surface plasmon resonance (SPR) market is expected to be the fastest-growing segment with a CAGR of 10.5% during the forecast period. Factors such as the increase in technological adoption in drug discovery and development processes, and real-time monitoring of biomolecular interactions are responsible for its popularity. In addition, the growing demand for high throughput screening methods, advanced techniques for analytics, and cost-effectiveness for drug discovery propel segment growth.
Regional Insights
The North America protein binding assays dominated the global market with a share of 40.8% in 2023. The increasing rate of chronic diseases in the population and government initiatives in countries such as the U.S., Canada, and Mexico contribute to its success. Increasing clinical trials, the rising number of biosimilars and biologics, and the demand for efficient and effective biologics are responsible for the dominance and growth of the market. In addition, the strengthening association between CROs and pharmaceutical companies further boosts market development.
U.S Protein Binding Assays Market Trends
The U.S protein binding assays is a dominating market in the North American region with a share of 78.0% in 2023. Factors such as favorable policies, emphasis on creating novel drugs, and drug efficacies, and participation of CROs and biopharmaceuticals encourage market growth. In addition, the growing initiatives by the government in support of biologics development, and the presence of major pharmaceutical players with innovative strategies further boost the market developments.
Europe Protein Binding Assays Market Trends
Europe protein binding assays market is expected to witness a significant growth in the coming years owing to the factors such as development in pharmacological assays, and discovery of a wide range of drug molecules and receptors are the key factors for the market growth. With the increasing adoption of protein-based drugs and the availability of advanced technologies for protein binding the heavy investments by major players are further boosting the market.
The UK protein binding assays market growth is highly anticipated over the forecast period as government initiatives and technological bases for drug discovery and development catalyze the clinical industry. Besides, the presence of advanced medical technology and heavy investment by the biologics organizations in protein binding have shaped the regional market.
Asia Pacific Protein Binding Assays Market Trends
Asia Pacific protein binding assays market is expected to be the fastest-growing with a CAGR of 11.1% over the forecast period. The increasing number of CRO operations, increasing expenditure for drug discovery, and increasing healthcare expenditure in countries such as China, India, and Japan. Additionally, the rise in research activities by the major players, and the growing interest in molecular technology supplements the regional market developments.
Key Protein Binding Assays Company Insights
Some major players in the protein binding assays market include Thermo Fisher Scientific, Adari Cell Sciences, Sartorius AG, and others. Organizations focus on increasing customer base to gain a competitive edge in the industry. Therefore, key players are undertaking several strategic initiatives, such as mergers and acquisitions, and partnerships with other major companies.
-
Thermo Fisher Scientific is a multinational clinical research and life science company that offers a comprehensive range of products and services for protein binding assays ranging from basic protein quantification to complex protein interactions.
-
Beckman Coulter primarily focuses on clinical diagnostics and research instruments that can be used in protein binding strategies. The key areas of relevance are protein chemistry analyzers, flow cytometry, and centrifugation.
Key Protein Binding Assays Companies:
The following are the leading companies in the protein binding assays market. These companies collectively hold the largest market share and dictate industry trends.
- Thermo Fisher Scientific
- ADMEcell, Inc.
- Beckman Coulter, Inc.
- Sartorius AG
- Bio-Rad Laboratories, Inc.
- Abzena Ltd
- Abcam Limited
- GVK Biosciences Private Limited
- Promega Corporation
- Arryait Corporation
- Charles River Laboratories
- Sovicell
- Eurofins Scientific
- 3B Pharmaceuticals
- Creative Biolabs
- Evotec SE
- BioDuro-Sundia
- Merck KDaA
Recent Developments
-
In May 2024, Beckman Coulter received a clearance from the U.S. Food and Drug Administration for its Access NT-proBNP assay using the Beckman Coulter Dxl 9000 Immunoassay Analyzer that assesses heart failure in less than 11 minutes.
-
In July 2023, Charles River Laboratories announced the acquisition of SAMDI tech. Under this, the companies offer end-to-end drug discovery portfolio, data generation, and fast identification for new drug discovery by combining their platform with premier. The platforms combined accelerate the client’s drug discovery efforts.
Protein Binding Assays Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 500.4 million
Revenue forecast in 2030
USD 896.3 million
Growth Rate
CAGR of 10.2% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Technology, products & services, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; South Korea; Australia; Brazil; Argentina; Saudi Arabia; UAE; Kuwait, South Africa
Key companies profiled
Thermo Fisher Scientific Inc.; ADMEcell, Inc.; Beckman Coulter, Inc.; Sartorius Stedim; BioOutsource Limited; Bio-Rad Laboratories, Inc.; Abzena Ltd; Abcam plc; GVK Biosciences Private Limited; Promega Corporation; Arrayit Corporation; Charles River Laboratories; Sovicell GMBH; Absorption Systems LLC; Eurofins Scientific; 3B Pharmaceuticals; Creative Biolabs; Evotec (Cyprotex); Bioduro; Merck KGaA
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
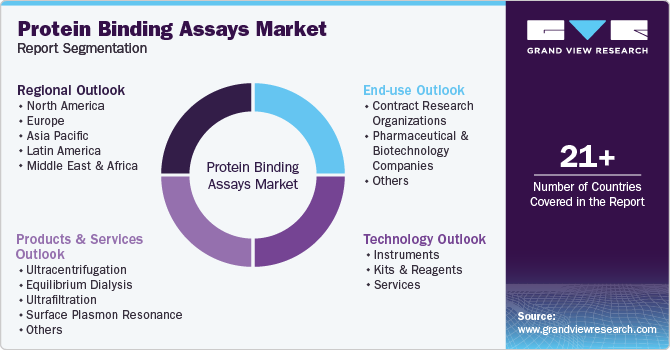
Global Protein Binding Assays Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global protein binding assays market report based on technology, products & services, end use, and region.
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
Instruments
-
Kits & Reagents
-
Services
-
-
Products & Services Outlook (Revenue, USD Million, 2018 - 2030)
-
Ultracentrifugation
-
Equilibrium Dialysis
-
Ultrafiltration
-
Surface Plasmon Resonance
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Contract Research Organizations
-
Pharmaceutical & Biotechnology Companies
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Spain
-
Italy
-
Denmark
-
Norway
-
Sweden
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
Saudi Arabia
-
UAE
-
Kuwait
-
South Africa
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










