- Home
- »
- Medical Devices
- »
-
Suture Anchor Devices Market Size, Industry Report, 2030GVR Report cover
![Suture Anchor Devices Market Size, Share & Trends Report]()
Suture Anchor Devices Market (2025 - 2030) Size, Share & Trends Analysis Report by Product (Absorbable, Non-absorbable), By Material, By Tying Type, By End-use (Hospitals, Ambulatory Surgical Centers, Clinics), By Region, and Segment Forecasts
- Report ID: GVR-4-68040-163-6
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Suture Anchor Devices Market Summary
The global suture anchor devices market size was valued at USD 815.6 million in 2024 and is projected to reach USD 1,003.4 million by 2030, growing at a CAGR of 3.7% from 2025 to 2030. This expansion is projected to be aided by a rising prevalence of sports injuries that has led to an increase in the demand for suture anchor devices.
Key Market Trends & Insights
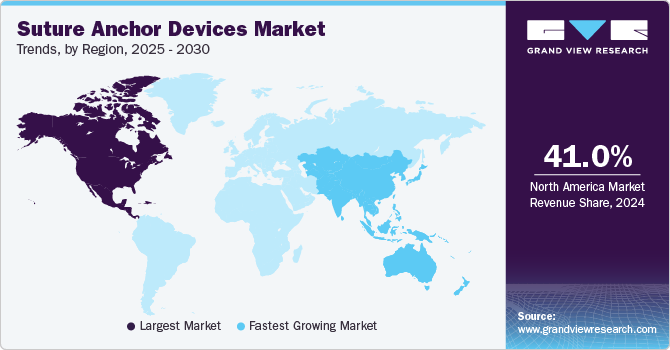
- North America led the overall suture anchor devices market in 2024, with the largest revenue share of 41.0%.
- Asia Pacific is expected to advance with the fastest growth rate during the forecast period, with a CAGR of 4.6%.
- Based on products, non-absorbable products led the market with the largest revenue share of 55.1% in 2024.
- In terms of tying type, the knotless segment dominated the market with the largest revenue share in 2024.
- Based on end-use, the hospitals segment accounted for the largest market revenue share in 2024.
Market Size & Forecast
- 2024 Market Size: USD 815.6 Million
- 2030 Projected Market Size: USD 1,003.4 Million
- CAGR (2025-2030): 3.7%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
Moreover, advancements in surgical techniques and materials incorporated into these devices have significantly contributed to their widespread adoption. Additionally, the rising aging population worldwide, along with prevalent orthopedic conditions, are some of the factors driving market expansion.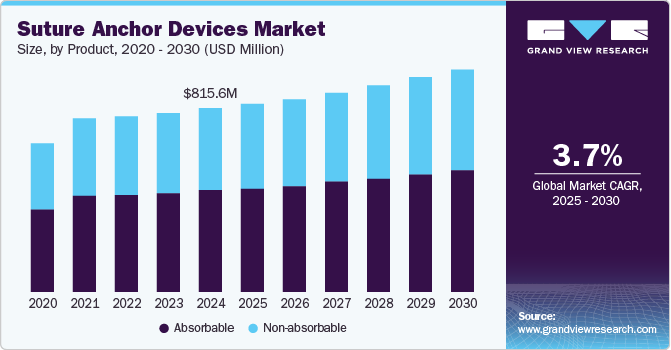
In sports medicine and arthroscopic surgery, suture anchors play a crucial role in anchoring soft tissue, including tendons, ligaments, and meniscus, to the bone. For instance, the number of injuries among players under the age of 21 in the Premier League escalated from 34 to 51 during the 2023-24 season. This rise is ascribed to the increasing physical demands imposed on these athletes and the congested fixture schedules they are required to navigate. Such trends underscore the increasing necessity for effective injury prevention and management strategies, particularly with respect to football-related injuries.
The market is also significantly influenced by the rising aging population, who are susceptible to musculoskeletal disorders, joint diseases, and soft tissue injuries. This has increased the demand for effective orthopedic solutions such as suture anchors, to repair affected ligaments and tendons in aging patients. As per the World Health Organization (WHO), the population aged 60 years and above is projected to increase from 1 billion in 2020 to 1.4 billion by 2030 and more than double to 2.1 billion by 2050. This demographic shift led to a surge in the conduction of orthopedic procedures among the elderly population, addressing conditions such as rotator cuff injuries and joint degeneration. Increase in such procedures are directly influencing the growing adoption and utilization of suture anchor devices to enhance the quality of life for the aging population.
The development of advanced suture anchor devices & surgical techniques is further driving the robust market growth of the suture anchor devices industry. Musculoskeletal injuries, particularly those affecting the shoulders, knees, and ankles, require surgical intervention to restore function and mobility. Suture anchors are essential in these procedures, providing secure fixation for tissues and bones during repair. Hence, the market is witnessing a rise in the adoption of minimally invasive surgeries, technological advancements, and research in suture anchor devices, further propelling its expansion. Additionally, a wide product range in the pipeline and a high market potential in untapped emerging economies are anticipated to serve as attractive opportunities for suture anchor devices during the forecast period.
Market Concentration & Characteristics
Regulatory standards, such as FDA (Food and Drug Administration) approvals in the U.S. or CE marking in the European Union, regulate the standards of surgical devices and treatments. Such regulation ensures comprehensive preclinical and clinical assessments, stringent manufacturing practices, and necessary certification for the product to be used in regular practice.
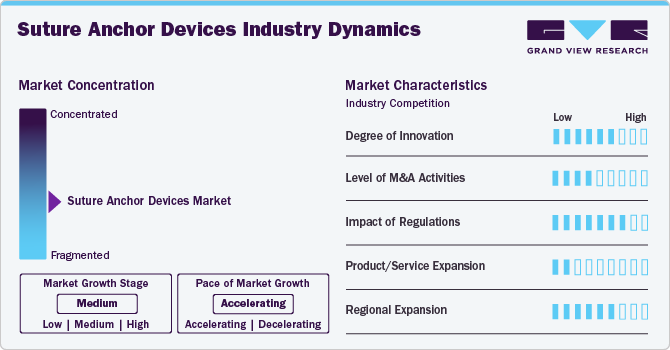
The market for suture anchor devices is subject to comprehensive regulations characterized by stringent guidelines that oversee product safety, effectiveness, and manufacturing procedures. Only a limited number of companies possess the capability to fulfill such stringent requirements and have the expertise required to achieve high manufacturing standards. Owing to this, the suture anchor devices industry is moderately consolidated with limited companies participating in the regional and global market. Smaller enterprises find it difficult to comply with the regulatory criteria or may lack the capacity to invest in the elaborate approval procedures. This scenario leads to a competitive environment in which major players hold a beneficial advantage, thereby restricting opportunities for new market entrants and making the market concentrated.
Product Insights
Non-absorbable products led the market with the largest revenue share of 55.1% in 2024. Dominance is aided by their use in orthopedic procedures to secure sutures in bone and maintain their structural integrity. They are designed to provide long-term fixation for ligament and tendon repairs and are commonly composed of polyester, polyethylene, and nylon. They offer reliable and long-lasting fixation, contributing significantly to their widespread utilization across various medical applications. The inherent durability and non-degradable properties of non-absorbable anchors play a critical role in achieving stability during recovery. Their widespread use across various orthopedic surgeries has contributed significantly to their market dominance compared to absorbable suture anchors.
The absorbable segment is expected to show the fastest growth of 3.7% CAGR, as these products are designed to degrade over time within the body, eliminating the need for surgical removal. They offer convenience by negating the requirement for follow-up and intervention for anchor removal, thus helping reduce patient discomfort and healthcare costs. Enhanced patient convenience and considerable reduction in treatment costs make absorbable suture anchors an attractive alternative in procedures where long-term fixation is not required. These anchors are particularly advantageous in outpatient procedures, where minimizing the need for follow-up visits and subsequent surgeries is a key priority. Furthermore, advancements in biodegradable materials and the development of innovative formulations that facilitate rapid and efficient degradation are driving increased demand for absorbable suture anchors. Technological innovation is expected to drive further enhancements in the performance of these devices, leading to improved healing outcomes and a reduced risk of complications.
Tying Type Insights
The knotless segment dominated the market with the largest revenue share in 2024. These anchors simplify the surgical process by eliminating the need for manual knot tying, reducing procedure time and potential complications associated with knot tying. They offer enhanced tissue preservation and distribute tension evenly across the repaired tissue, improving outcomes and potentially resulting in faster patient rehabilitation. Consistent tension distribution is essential for proper tissue healing, as knot-related inconsistencies offer uniform tension across the repair site.
The knotted sutures segment is expected to experience significant growth due to their ongoing utilization in specific orthopedic and surgical procedures where knotting is the preferred technique for securing tissue. The demand for knotted sutures is propelled by the dependability and familiarity associated with traditional knot-tying techniques that enhance surgeons' control over tension and flexibility during complex repairs. Furthermore, knotted sutures are more economical compared to knotless alternatives, rendering them a preferred choice in markets where cost factors are critical. Knotted sutures maintain their value compared to knotless alternatives, particularly in procedures where precision and control are paramount.
End-use Insights
The hospitals segment accounted for the largest market revenue share in 2024. Hospitals conduct more orthopedic procedures, including those involving suture anchors for ligament and tendon repairs. The specialized orthopedic departments and resources required for complex orthopedic surgeries contribute to the significant usage of suture anchor devices in these facilities. Factors such as the increasing number of surgeries performed in hospitals, the establishment of multi-specialty hospitals worldwide, and the rapid adoption of more advanced biosurgery treatment procedures have further fostered the suture anchor devices industry.
Hospitals are prioritizing patient care and surgical outcomes, leading to a surge in demand for suture anchor devices. This is due to the adoption of minimally invasive techniques, which reduce recovery times and enhance patient outcomes. The growth of hospital networks, especially in emerging markets with improved healthcare access, is also boosting the market as hospitals expand their orthopedic departments to meet the increasing demand for orthopedic surgeries. Additionally, the rising geriatric population, increasing incidences of knee injuries, and rapid adoption of advanced orthopedic surgical equipment have also contributed to hospitals being the leading end-use segment.
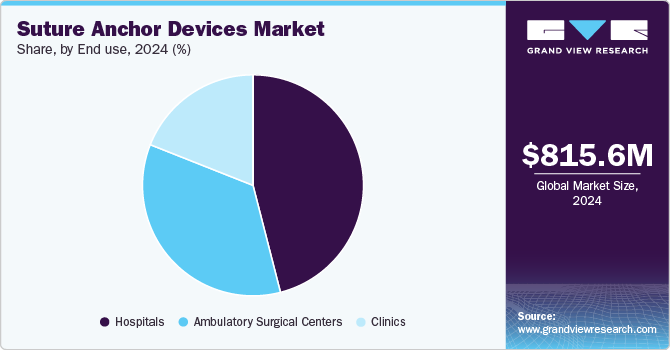
The ambulatory surgical centers segment is estimated to register the fastest CAGR over the forecast period. Ambulatory surgical centers have gained substantial popularity due to their cost-effectiveness, efficiency in performing minor surgeries, and advancements in medical technologies, allowing more procedures to be performed outside traditional hospital settings. Additionally, the shift toward outpatient procedures for orthopedic surgeries has increased the demand for suture anchor devices in these centers.
Regional Insights
North America led the overall suture anchor devices market in 2024, with the largest revenue share of 41.0%. This dominance can be attributed to several key factors, including the region's advanced healthcare landscape and its capacity to support the widespread adoption of innovative medical technologies, including suture anchor products. The presence of a large patient population, favorable reimbursement policies in the healthcare system, and a higher number of research and development activities are also contributing to the market growth. Furthermore, North America is a hub for research and development (R&D) activities in the medical device industry. Leading companies in the suture anchor industry are based in the U.S., thus driving continuous innovation and technological advancements in anchor design, materials, and performance. Key players such as Smith & Nephew, Stryker Corporation, and Johnson & Johnson are headquartered in North America, contributing to the region's market dominance. Moreover, the increasing number of surgeries performed in hospitals, the establishment of multi-specialty hospitals, and the rapid adoption of more advanced biosurgery treatment procedures have all contributed to North America's dominance in the market for suture anchor devices.
U.S. Suture Anchor Devices Market Trends
The U.S. accounted for North America's largest share in suture anchor devices in 2024. The country's ability of patients to afford medical treatment and a rise in the adoption rate of suture anchor products are the major factors contributing to market growth. Additionally, the high prevalence of sports-related injuries has prompted a substantial demand for orthopedic interventions, stimulating the usage of suture anchors in repairing ligament and tendon injuries.
In adults aged above 75 years, hip fractures become the most common injury area. Fractures account for 16% of all musculoskeletal injuries in the U.S. annually. Over 40% of fractures take place at home. These factors collectively set the U.S. as the leading country in the suture anchor device market, with the highest revenue share and a strong outlook for continued dominance.
Furthermore, the demand for precise treatments in complex surgeries is rising due to the growing focus on personalized surgical solutions. The expansion of outpatient surgical centers and the trend toward early discharge from hospitals have further increased the demand for these devices. Moreover, the U.S. has a favorable regulatory environment, with medical device advances subject to rigorous yet rapid clearance processes. The FDA's quick clearance of new goods and the country's significant clinical research and testing have resulted in the development and widespread use of cutting-edge suture anchor devices. This regulatory framework and a culture of early technological adoption place the U.S. as a leader in the suture anchor industry.
Europe Suture Anchor Devices Market Trends
The suture anchor devices market in Europe is projected to grow at a considerable CAGR from 2025 to 2030. The demand for suture anchor devices in Europe is rising due to several factors. There is an elderly population that results in more joint issues and musculoskeletal disorders, and this is amplifying the need for effective therapies. Sports injuries also elevate the demand, particularly among active youth. The shift towards minimally invasive surgery promotes the use of suture anchors, as they facilitate smaller incisions and quicker recovery. The inclination for arthroscopic surgery is also on the rise, as it inflicts less tissue harm and diminishes complications.
Moreover, surgeons in Europe are receiving training in advanced, less invasive methods, resulting in greater utilization of suture anchor devices. Educational programs and workshops on these technologies are also on the rise. Furthermore, improved reimbursement policies for orthopedic surgeries are helping patients afford treatments using these devices which is helping in boosting market growth. Innovative materials, such as bio-absorbable alternatives, boost the dependability of these devices, making them increasingly favored in surgical procedures. Altogether, these elements highlight the dynamic characteristics of the economy and its adaptability to shifting technological advancements and healthcare requirements.
Asia Pacific Suture Anchor Devices Market Trends
Asia Pacific is expected to advance with the fastest growth rate during the forecast period, with a CAGR of 4.6%. The rising geriatric population increases the incidence of orthopedic conditions, necessitating more interventions involving suture anchors. Furthermore, the Asia-Pacific region is experiencing a substantial movement toward expanding healthcare access in rural and disadvantaged areas. The spread of healthcare services into these areas and a growing middle class are driving up demand for modern medical procedures, such as orthopedic surgery. This tendency, particularly in countries with huge populations such as India and China, has been critical in promoting suture anchor devices since the number and diversity of procedures needing these devices continue to rise. Moreover, continuous technological advancements, increased awareness about advanced medical treatments, and expanding investments in healthcare contribute to the anticipated rapid growth rate of the market for suture anchor devices in the Asia Pacific region.
The rise in sports participation is not confined to established markets such as North America and Europe. It is extending to emerging economies in Asia-Pacific. The countries in the region continue to develop and enhance their healthcare infrastructure, and there is a growing demand for advanced orthopedic solutions, including suture anchor devices. This is particularly evident as sports injuries in these regions are increasingly managed through advanced surgical techniques.
Japan accounted for the largest regional share in the market for suture anchor devices in 2024. The economy has witnessed a surge in technological advancements and research in the field of suture anchor devices. Additionally, Japan's efficient and transparent regulatory framework for medical devices encourages the development and approval of new suture anchor products. The country is home to several leading orthopedic companies, such as DePuy Synthes and Arthrex, which have a strong presence in the market. These factors collectively contribute to Japan's leading position in the Asia Pacific market for suture anchor devices, reflecting the region's robust growth and potential for further expansion.
Key Suture Anchor Devices Company Insights
Some of the dominant players operating in the market for suture anchor devices include Smith & Nephew plc; Zimmer Biomet Holdings Inc.; CONMED Corporation; Arthrex, Inc.; Johnson and Johnson (DePuy Synthes, Inc.); Stryker Corporation; Wright Medical; and OrthoMed.
-
Smith & Nephew plc is a global medical product company specializing in orthopedic reconstruction, sports medicine, and wound management. It is recognized for its extensive array of orthopedic products, including advanced joint replacement systems, which have augmented its footprint in both emerging and established markets. Their sports medicine division, which emphasizes minimally invasive surgical solutions, drives significant growth in arthroscopic procedures, especially for tendon and ligament repairs.
-
Zimmer Biomet Holdings Inc. offers innovative musculoskeletal healthcare solutions, offering a broad spectrum of products and services in orthopedics, spine, dental implants, and more. The company’s concentration on personalized care and advancements in robotics and 3D printing technologies for joint replacements contribute considerable value to its product offerings. Their emphasis on enhancing surgical solutions, particularly regarding patient-specific products such as custom implants, facilitates better surgical outcomes and quicker recovery periods.
-
CONMED Corporation operates in over 80 countries worldwide. The company focuses on minimally invasive surgical devices and equipment for arthroscopy, providing solutions for orthopedic surgeries and sports medicine. This focus has resulted in the creation of state-of-the-art minimally invasive surgical instruments, improving accuracy and reducing patient recovery periods. The firm’s commitment to delivering superior arthroscopic equipment has established it as a reliable collaborator for orthopedic surgeons across the globe.
Meanwhile, Parcus Medical and Enovis Corporation are some of the emerging players operating in the suture anchor device market.
-
Parcus Medical is a medical device company that offers over 400 innovative products in more than 60 countries worldwide, with a direct selling presence in countries outside the U.S. Their global presence, coupled with an emphasis on innovation and surgical solutions, places them in a strong position for sustained growth in the competitive medical device industry.
-
Enovis Corporation is a medical device company specializing in developing orthopedic implants and instruments for spine surgery. It has positioned itself as a prominent entity in designing and developing these implants and instruments. Enovis Corporation focuses on improving surgical outcomes and patient recovery durations.
Key Suture Anchor Devices Companies:
The following are the leading companies in the suture anchor devices market. These companies collectively hold the largest market share and dictate industry trends.
- Smith & Nephew, plc.
- Zimmer Biomet Holdings, Inc.
- CONMED Corporation
- Arthrex, Inc.
- Johnson & Johnson Services, Inc. (DePuy Synthes, Inc.)
- Stryker (Wright Medical Group, Inc.)
- Parcus Medical, LLC (Medacta International)
- OrthoMed, Inc.
- Enovis Corporation
- Medtronic
Recent Developments
-
Since May 2024, nine suture anchor devices have been in various stages of development globally, with six in the early stages and two in the late stages. These innovations aim to enhance the effectiveness of soft tissue fixation in orthopedic surgeries. Key players engaged in the ongoing advancement of Suture Anchors comprise KeriMedical, Magnesium Development Company, Parcus Medical, Parcus Medical, San Francisco VA Medical Center, SINTX Technologies, University of Pittsburgh, DePuy Synthes, and Hospital for Special Surgery.
-
In September 2023, at the 2023 Orthopaedic Summit (OSET) Annual Meeting, Anika Therapeutics, Inc. announced the launch of their RevoMotion Reverse Shoulder Arthroplasty System (RSA). The company also showcased the Integrity Implant System and the X-Twist Fixation System at the event. Anika's advanced shoulder portfolio, which includes the recently introduced X-Twist suture anchors, aligns with the increasing demand for suture anchor devices in the orthopedic market.
-
In December 2022, Stryker launched Citrefix, a suture anchor system for ankle and foot surgical procedures, leveraging Citregen biomaterial that is designed to support bone regeneration and the natural healing process. Citregen, a bioresorbable substance, has been designed to emulate native bone structure and chemistry.
Suture Anchor Devices Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 837.9 million
Revenue Forecast in 2030
USD 1,003.4 million
Growth Rate
CAGR of 3.7% from 2025 to 2030
Base Year for Estimation
2024
Historical Data
2018 - 2023
Forecast Period
2025 - 2030
Report updated
April 2025
Quantitative Units
Revenue in USD million and CAGR from 2025 to 2030
Report Coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments Covered
Product, material, tying type, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait; Romea
Key companies profiled
Smith & Nephew, plc., Zimmer Biomet Holdings, Inc., CONMED Corporation, Arthrex, Inc., Johnson & Johnson Services, Inc. (DePuy Synthes, Inc.), Stryker (Wright Medical Group, Inc.), Parcus Medical, LLC (Medacta International), OrthoMed, Inc., Enovis Corporation, Medtronic
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Suture Anchor Devices Market Report Segmentation
This report forecasts revenue growth at the global, regional & country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global suture anchor devices market report on the basis of product, material, tying type, end use, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Absorbable
-
Non-absorbable
-
-
Material Outlook (Revenue, USD Million, 2018- 2030)
-
Metallic Suture Anchor
-
Bio-absorbable Suture Anchor
-
Others
-
-
Tying Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Knotted Suture Anchors
-
Knotless
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Ambulatory Surgical Centers
-
Clinics
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










