- Home
- »
- Pharmaceuticals
- »
-
Transthyretin Amyloidosis Treatment Market Size Report, 2030GVR Report cover
![Transthyretin Amyloidosis Treatment Market Size, Share & Trends Report]()
Transthyretin Amyloidosis Treatment Market Size, Share & Trends Analysis Report By Type (ATTR-PN, ATTR-CM), By Therapy, By Disease Type, By Distribution Channel, By Region, And Segment Forecasts, 2022 - 2030
- Report ID: GVR-4-68039-977-9
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2020
- Forecast Period: 2022 - 2030
- Industry: Healthcare
Report Overview
The global transthyretin amyloidosis treatment market size was valued at USD 4.72 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 7.6% from 2022 to 2030. The growth is attributed to the extensive research in the development of new therapies and the presence of robust pipeline drugs that are used to treat transthyretin. It is a rare and progressive disease characterized by the deposition of abnormal proteins such as misTTR in organs and tissue of the body. The symptoms associated with the disease mainly depend on the organs that are involved such as carpel syndrome for arms, lumbar spinal stenosis for the back, vitreous opacities for the head and neck, and swelling at the feet. There are two major types of transthyretin amyloidosis such as mutated/ hereditary ATTR and wild-type ATTR. It often affects major organs of the body such as the liver, kidney, nerves, and heart. However, the unavailability of advanced diagnostic facilities in developing countries is a major challenge to the transthyretin amyloidosis treatment market growth.
The COVID-19 pandemic negatively impacted the market growth owing to the decrease in a clinical trials for the drugs that could be used for the transthyretin amyloidosis treatment. For instance, in September 2020, an article published in the NCBI stated that a 74% of reduction has been witnessed in the average number of patients entering clinical trials. Patients suffering from transthyretin amyloidosis have a higher risk of mortality and morbidity owing to their age and ATTR- amyloidosis-related organ dysfunction. Therefore, proper characterization of risk and management implications are needed for the patients if they develop COVID-19. The implications of delayed diagnosis and interrupting treatment of patients should be balanced considering the risk of exposure to the virus in the healthcare setting.
The increasing prevalence of transthyretin amyloidosis is anticipated to fuel market growth. Based on the article published on Prothena, it is estimated that around 50,000 people live with hATTR amyloidosis and about 400,000 people live with ATTRwt amyloidosis worldwide. The growing geriatric population in the country is anticipated to increase the prevalence of transthyretin amyloidosis over the forecast period. As per the NCBI data, an age-related form of amyloidosis primarily affects the heart and is also known as wild-type ATTR. For instance, in 2020, around 727 million people globally were aged 65 years and above. The geriatric population worldwide is growing substantially at a rate of 16.0% and is expected to reach 1.5 billion in 2050. Aging is considered a substantial risk factor for wild types of amyloidosis (ATTRwt).
Initiatives undertaken by government and non-government bodies for creating awareness regarding the disease and its treatment benefits among people are expected to boost early diagnosis of disease, thereby increasing the treatment rate. For instance, in May 2021, the Australian Amyloidosis Network organized a biennial touring workshop for creating awareness about the disease among health professionals and patients with ATTR amyloidosis. These workshops support the physicians and patients in understanding the disease’s complexity, which will further help in developing new therapies for the treatment of transthyretin amyloidosis.
Type Insights
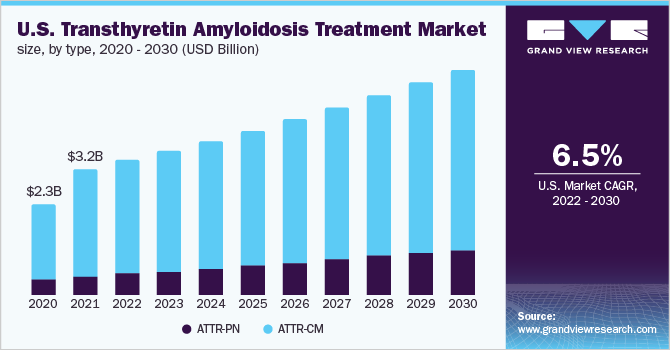
ATTR-CM dominated the market for transthyretin amyloidosis and accounted for a revenue share of more than 80.0% in 2021. This is owing to the high prevalence of disease and improving diagnosis rates at a significant pace. The exact prevalence of the disease is unknown, but various autopsy studies suggest that around 22% to 25% of people aged 80 years and above reported TTR amyloid deposition in the heart. Although in most cases, the degree of amyloid deposition is mild among ATTR-CM amyloidosis patients. Moreover, the launch of new drugs for the treatment of ATTR-CM is expected to boost segment growth. For instance, in May 2019, Pfizer Inc. received approval for its VYNDAQEL and VYNDAMAX from the U.S. FDA for the treatment of ATTR-CM.
ATTR-PN is estimated to register the highest growth rate over the forecast period. The growth of the segment is attributed to the high incidence rate and approval of new drug therapies for the treatment of ATTR-PN amyloidosis patients. Val30Met is the most common TTR mutation found in patients with neuropathy. Inotersen and Patisiran are the two FDA-approved drugs available for the treatment of ATTR-PN in the market. Both drugs inhibit the production of TTR in the liver and have shown to be effective in ATTR-PN. Furthermore, the fast-track approval of products worldwide is expected to boost segment growth. For instance, in June 2019, the Ministry of Health, Labor, and Welfare of Japan approved ONPATTRO (Patisiran) for the treatment of hATTR in adults.
Disease Type Insights
Hereditary transthyretin amyloidosis captured the largest revenue share of over 60.0% in 2021. Hereditary transthyretin-mediated amyloidosis is an autosomal dominant, rare, and fatal disease in which it impairs multiple organs, leading to death and disability. To overcome this situation, pharmaceutical companies are constantly working on drug development and several drugs are still in pipeline for commercialization. For instance, in August 2022, Attralus, Inc. received U.S. FDA authorization of orphan drug designation for 124I-AT-01, which could be used for the diagnosis of transthyretin amyloidosis.
Furthermore, several countries are anticipated to witness a higher number of cardiomyopathy cases. Therefore, intensive R&D is happening in the market for the proper diagnosis and treatment of the disease. For instance, in August 2022, Alnylam Pharmaceuticals, Inc. received FDA authorization for the drug Onpattro, which could be used for nerve pain treatment caused by hereditary transthyretin amyloidosis. Moreover, its phase 3 data shows that it has the potential to help patients suffering from cardiac-related issues for rare protein diseases.
Distribution Channel Insights
Hospital pharmacies captured the largest revenue share of over 30.0% in 2021. Most hospitals are working toward the diagnosis, research, and treatment of the disease. For instance, Brigham and Women’s Hospital in Massachusetts, U.S. focuses on diagnosing and treating misdiagnosed diseases. It has established the Amyloidosis Program, which offers access to innovative therapies, patient-centered care, and new clinical trials. The team of experts would educate physicians and patients and spread awareness among the patients. These initiatives would further contribute to the segment's growth.
Online pharmacies are anticipated to witness the fastest growth in the forecast period owing to the easy access of drugs to patients, which is also expanding the use to get health information. However, there is an increasing risk of patients purchasing products from illegal websites is expected to hamper the growth. For instance, an online publication stated that fraudulent online pharmacies are attempting to sell illegal generic versions of Vyndamax. This could be potentially unsafe for the customers. Therefore, promotional campaigns would further spread awareness among the public about safe and authentic online pharmacies, which could further prevent safety threats to patients.
Therapy Insights
The targeted therapy segment dominated the market and accounted for a revenue share of over 90.0% in 2021. Drugs such as Onpattro, Inotersen, and Tafamidis are included in this segment. Onpattro is administrated intravenously to ATTR-PN patients at an interval of 3 weeks with pretreatment of steroids and Benadryl to avoid side effects. Onpattro works by silencing the portion of RNA involved in producing TTR protein. Moreover, an increasing prevalence of the disease ATTR-PN and worldwide approval of these therapies are projected to fuel segment growth. For instance, in July 2018, the Food and Drug Administration (FDA) approved Onpattro (Patisiran) developed by Alnylam Pharmaceuticals, Inc. for the treatment of ATTR-PN in adults. Along with this, it holds the commercialization rights to Patisiran in Canada, the U.S., and Western European countries, while Sanofi Genzyme holds the right to the rest of the world.
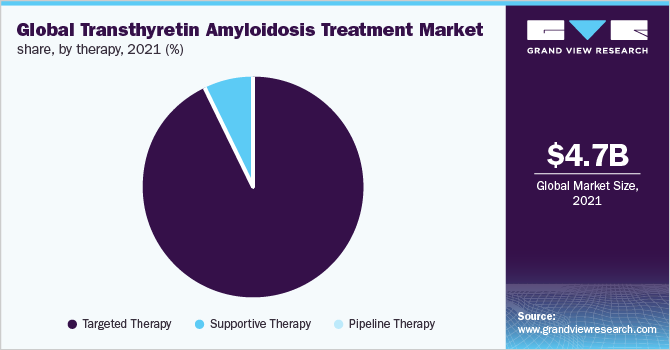
Targeted therapy is expected to witness the fastest growth during the forecast period owing to increasing initiatives undertaken by key players to support the patients. For instance, Akcea Therapeutics, Inc. operates a patient assistance program “Akcea Connect” to support hATTR amyloidosis patients. Under this program, the eligible person and their families get free, private, and personalized support across the U.S. This will increase awareness about available drug therapies, encouraging general practitioners to prescribe these drugs to transthyretin amyloidosis patients. Hence, the high prescription rate and the high cost of these drugs are major factors fueling the targeted therapy segment growth.
Regional Insights
North America dominated the market with a revenue share of over 65.0% in 2021 and the region is anticipated to maintain its dominance during the forecast period. Increasing novel drugs prescription, proper reimbursement policies, and higher treatment rates in the region are factors supporting transthyretin amyloidosis treatment market growth in the region. The introduction of new therapies in the region is acting as a key market driver. For instance, in July 2019, Health Canada approved ONPATTRO for the treatment of hATTR with polyneuropathy in adults. This approval is projected to have a positive impact on the regional market.
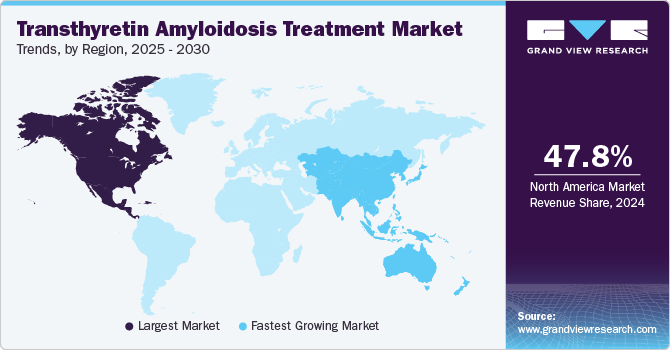
The MEA region is projected to witness the fastest growth over the forecast period. The high unmet treatment needs, increasing prevalence of disease, and rising approval are expected to drive the market in the coming years. Moreover, major factors such as a large target population, good healthcare reforms, and an increase in disease awareness are expected to be the primary growth factors.
Key Companies & Market Share Insights
Several market players are working toward the development and commercialization of drugs for the proper diagnosis and treatment of transthyretin amyloidosis. For instance, in January 2022, AstraZeneca’s drug Eplontersen received Orphan Drug Designation in the U.S. by the FDA for the transthyretin-mediated amyloidosis treatment. Similarly, in June 2022, Alnylam Pharmaceuticals, Inc. received FDA authorization for AMVUTTRA (vutrisiran) for the treatment of Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis. Continuous advancements by the market players would further drive the market during the forecast period. Some prominent players in the global transthyretin amyloidosis treatment market include:
-
Pfizer Inc.
-
Johnson & Johnson Services, Inc.
-
Ionis Pharmaceuticals, Inc.
-
Alnylam Pharmaceuticals, Inc.
-
BridgeBio Pharma, Inc.
-
Bristol-Myers Squibb Company
-
Acrotech Biopharma
-
AstraZeneca
-
Astellas Pharma, Inc.
-
Prothena
-
SOM Biotech
Transthyretin Amyloidosis Treatment Market Report Scope
Report Attribute
Details
Market size value in 2022
USD 5.11 billion
Revenue forecast in 2030
USD 9.17 billion
Growth rate
CAGR of 7.6% from 2022 to 2030
Base year for estimation
2021
Historical data
2018 - 2020
Forecast period
2022 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2022 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, therapy, disease type, distribution channel, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Russia; Japan; India, China, Australia; Singapore, Brazil; Mexico; Argentina, South Africa, Saudi Arabia, UAE
Key companies profiled
Pfizer, Inc.; Johnson & Johnson Services, Inc.; Ionis Pharmaceuticals, Inc.; Alnylam Pharmaceuticals, Inc.; BridgeBio Pharma, Inc.; Bristol-Myers Squibb Company; Acrotech Biopharma; AstraZeneca; Prothena; SOM Biotech
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Transthyretin Amyloidosis Treatment Market Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global transthyretin amyloidosis treatment market report on the basis of type, therapy, disease type, distribution channel, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
ATTR-PN
-
ATTR-CM
-
-
Therapy Outlook (Revenue, USD Million, 2018 - 2030)
-
Targeted Therapy
-
Onpattro
-
Inotersen
-
Vyndaqel/Vyndamax
-
-
Supportive Therapy
-
Pipeline Therapy
-
-
Disease Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Hereditary Transthyretin Amyloidosis
-
Polyneuropathy
-
Cardiomyopathy
-
Mixed Type
-
-
Wild Type Amyloidosis
-
-
Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital Pharmacies
-
Specialty Pharmacies
-
Retail Pharmacies
-
Online Pharmacies
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
France
-
Italy
-
Spain
-
Russia
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Singapore
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
-
Frequently Asked Questions About This Report
b. The global transthyretin amyloidosis treatment market size was estimated at USD 4.72 billion in 2021 and is expected to reach USD 5.11 billion in 2022.
b. The global transthyretin amyloidosis treatment market is expected to grow at a compound annual growth rate of 7.6% from 2022 to 2030 to reach USD 9.17 billion by 2030.
b. North America dominated the transthyretin amyloidosis treatment market with a share of 68.20% in 2021. This is attributable to the rising prevalence of Transthyretin amyloidosis in the U.S., presence of well-established healthcare facilities, higher awareness levels amongst individuals, favorable reimbursement policies, and ample availability of treatment options.
b. Some key players operating in the transthyretin amyloidosis treatment market include Pfizer Inc., Johnson & Johnson Services, Inc., Ionis Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Inc., BridgeBio Pharma, Inc., Bristol-Myers Squibb Company, Acrotech Biopharma, AstraZeneca, Astellas Pharma, Inc., Prothena, and SOM Biotech.
b. Key factors that are driving the market growth include the increasing incidence of the disease, growing geriatric population, and increasing collaboration of research institutes with major players for the innovation of new target-specific therapies.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





