- Home
- »
- Pharmaceuticals
- »
-
Vulvovaginal Candidiasis Treatment Market Size Report, 2030GVR Report cover
![Vulvovaginal Candidiasis Treatment Market Size, Share & Trends Report]()
Vulvovaginal Candidiasis Treatment Market Size, Share & Trends Analysis Report By Drug Type (Clotrimazole, Fluconazole, Terconazole), By Route Of Administration, By Distribution Channel, By Region, And Segment Forecasts, 2022 - 2030
- Report ID: GVR-4-68039-997-1
- Number of Report Pages: 100
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2020
- Forecast Period: 2022 - 2030
- Industry: Healthcare
Report Overview
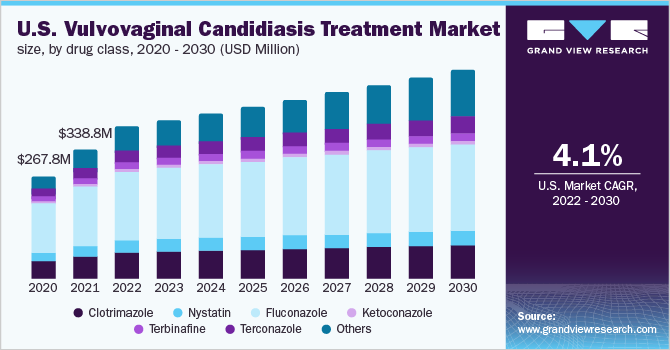
The global vulvovaginal candidiasis treatment market size was valued at USD 986.5 million in 2021 and is expected to grow at a compound annual growth rate (CAGR) of 4.39% from 2022 to 2030. According to an NCBI article, VVC is a common infection among women affecting around 138 million women annually worldwide. Strong emerging pipeline with recent approvals of therapies in disease management, rising disease burden, and launch of new drugs, such as BREXAFEMME and VIVJOA, are among the primary factors driving the industry growth. The global rise in VVC infection in women is such that it affects nearly 75% of women who report disease occurrence at least once in their lifetime.
According to an NCBI article, globally, complicated VVC affects around 10-20% of women and 9% of women report recurrent VVC cases. Furthermore, an upsurge in the prevalence of the disease is observed due to increasing diabetic patients and rising antibiotic resistance cases among individuals. It is also estimated that around 15% of such cases require special therapeutic considerations. Thus, the rising prevalence of the disease is driving industry growth. The increasing demand for better therapeutic options is leading to innovations in R&D, new approvals, and launches of drugs, thereby creating a lucrative opportunity for industry growth.
For example, in August 2022, VIVJOA an oral drug successfully received FDA approval. Mycovia Pharmaceuticals’ VIVJOA is an antifungal drug, which selectively inhibits fungal CYP5. In June 2021, Scynexis, Inc. announced the FDA approval for their novel drug BREXAFEMME (Ibrexafungerp) indicated for VVC treatment. All these launches are anticipated to fuel the industry's growth. Inflammasome, immunopathology, and PRR-mediated signaling at the vaginal mucosa are some of the recent advancements in the space. Such developments are offering lucrative opportunities for new therapeutic options.
Drug Class Insights
The fluconazole segment dominated the global industry in 2021 and accounted for the maximum share of the overall revenue. However, with the entry of BREXAFEMME and Mycovia in the space, Fluconazole share is expected to decline over the coming years. Nevertheless, these novel drugs come with higher pricing, which could slow down their market penetration. Antimycotic agent, Clotrimazole, was first registered under the brand name Canesten in Germany in 1973. Canesten is still among the leading brand for the treatment of vulvovaginal candidiasis. Later, external cream, internal vaginal cream, and soft capsule (soft ovule) were available. The industry has the presence of different formulations under different trade names, such as Abzorb, Candid-V, Mycoderm-C, Surfaz, Kansel, Imidil, Orasep OT, and Cloben.
In many countries, mono preparations of clotrimazole are available over the counter with a covered dose range of 100 to 500mg. Furthermore, the drug is also available in combinations, such as clotrimazole with fluconazole for effective management of the disease. All such factors support the industry's growth. FDA approved nystatin in 1971, which is sold under various brand names, such as Mycostatin, Bio-Statin, Pediaderm AF, Nystat, Nystop, and Nilstat. It is available in various forms, such as a tablet, cream, ointment, troches, and powder, for suspension. Nystatin has proved effective in fluconazole-resistant Candida. The drug mimics the immunoregulatory role by increasing the levels of IL-17 and IFN-gamma to improve antifungal immunity in the vagina. All these factors are anticipated to drive industry growth.
Route Of Administration Insights
The oral segment dominated the industry in 2021 and accounted for the maximum share of more than 42.30% of the total revenue. It is anticipated to maintain its share throughout the forecast period owing to the common & convenient route of administration for azoles, good bioavailability, and use for anti-fungal drugs. Oral fluconazole is the most common prescription drug recommended as treatment and maintenance therapy. It is also effective as short-duration therapy in recurrent VVC cases. Recently, the FDA has approved two oral dosage regimes of VIVJOA (oteseconazole) to reduce incidences of recurrent VVC. The topical segment is expected to grow at a steady CAGR during the study period.
A strong presence of topical products such as over-the-counter and prescription formulations boost the demand in the market. OTCs include Clotrimazole cream, Miconazole cream, and Tioconazole ointment, whereas Butoconazole cream and Terconazole cream are prescription formulations. Furthermore, topical antifungal recommendations also include econazole and nystatin in the market for more than 30 years. According to IDSA guidelines, uncomplicated VVC should be treated with topical antifungal agents. Furthermore, oral azoles are ineffective in treating. Glabrata vulvovaginitis, thereby, is prescribed for topical intravaginal boric acid or topical flucytosine cream.
Distribution Channel Insights
On the basis of distribution channels, the industry has been categorized into retail pharmacy, online pharmacy, and hospital pharmacy. The retail pharmacy segment is anticipated to witness the fastest CAGR of more than 4.70% over the forecast period. As disease treatment, control and management take short- as well as long-term support of medications, which boosts the segment growth. The market has both prescription and OTC drugs, thereby pharmacists play an important role by counseling patients and gaining their trust. Retail pharmacies are at ease for most medications, especially in the homecare setting. All these factors are expected to fuel the segment's growth.
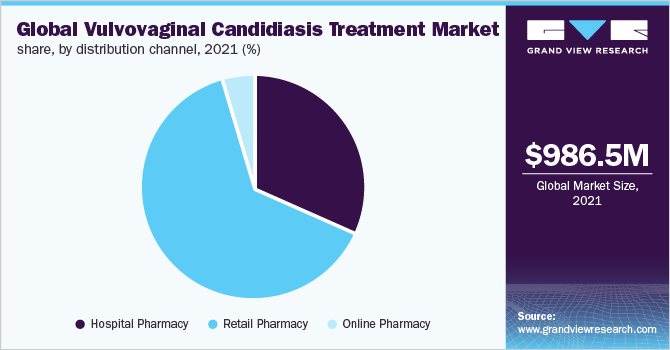
The online pharmacy distribution channel segment is also expected to grow at a significant CAGR during the forecast period.The preference for online purchasing is high due to the convenience and other benefits, such as home delivery. Amid the pandemic, people experienced the comfort of delivery services and reduced travel time for prescription fillin retail stores. The COVID-19 pandemic boosted the online segment due to the government restrictions like nationwide lockdowns and stay-at-home orders to curb the spread of the virus.
Regional Insights
On the basis of geographies, the industry has been categorized into North America, Asia Pacific, Latin America, Europe, Middle East & Africa. North America dominated the global industry in 2021 and accounted for the maximum share of more than 37.50% of the overall revenue. The region is expected to expand further at a steady growth rate maintaining its dominant position throughout the forecast period. This dominance can be attributed to the high disease prevalence, rise in patient awareness, increased healthcare expenditure, and the presence of major players in the region.
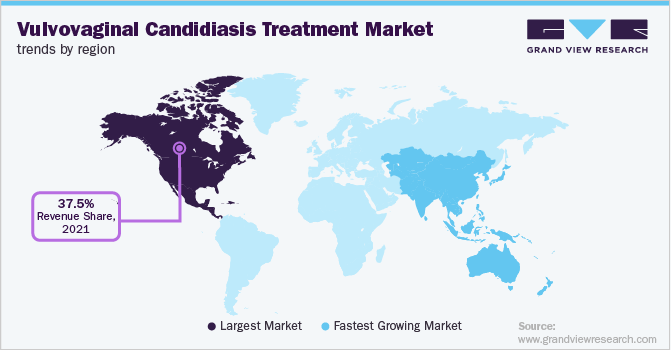
Advanced therapeutic options, new approvals, launches, and proactive government measures further contribute to regional market growth. On the other hand, Asia Pacific is estimated to witness the fastest growth rate over the forecast years. The high growth rate can be attributed to the rising disease burden of VVC and increasing testing rates. Positive changes, such as healthcare benefits by the government, increased awareness among consumers, and willingness to avail medical treatments are also expected to drive growth in the region.
Key Companies & Market Share Insights
Major players are undertaking various strategic initiatives, such as geographical expansion, merger, and acquisition, to gain a higher market share. Moreover, companies are focusing on gaining approvals for novel products that have the potential to address unmet needs. For instance, Mycovia Pharmaceuticals’ VIVJOA, which was already approved in some European, Asian, and Latin American countries, received FDA approval for recurrent VVC treatment in the U.S. in August 2022. Some of the key players in the global vulvovaginal candidiasis treatment market include:
-
Astellas Pharma Inc.
-
Mycovia Pharmaceuticals, Inc.
-
Basilea Pharmaceutica Ltd.
-
Scynexis, Inc.
-
Grupo Ferrer Internacional S.A.
-
Pfizer, Inc.
-
Cadila Pharmaceuticals
-
Bayer AG.
-
Bristol-Myers Squibb Company
Vulvovaginal Candidiasis Treatment Market Report Scope
Report Attribute
Details
Market size value in 2022
USD 1,162.5 million
Revenue forecast in 2030
USD 1.64 billion
Growth rate
CAGR of 4.39% from 2022 to 2030
Base year for estimation
2021
Historical data
2018 - 2020
Forecast period
2022 - 2030
Quantitative units
Revenue in USD million and CAGR from 2022 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Drug class, route of administration, distribution channel, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; U.K.; France; Italy; Spain; China; Japan; India; Australia; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE
Key companies profiled
Astellas Pharma Inc.; Mycovia Pharmaceuticals, Inc.; Bayer AG.; Basilea Pharmaceutica Ltd.; Scynexis, Inc.; Grupo Ferrer Internacional S.A.; Pfizer, Inc.; Cadila Pharmaceuticals; Bristol-Myers Squibb Company
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Vulvovaginal Candidiasis Treatment Market Segmentation
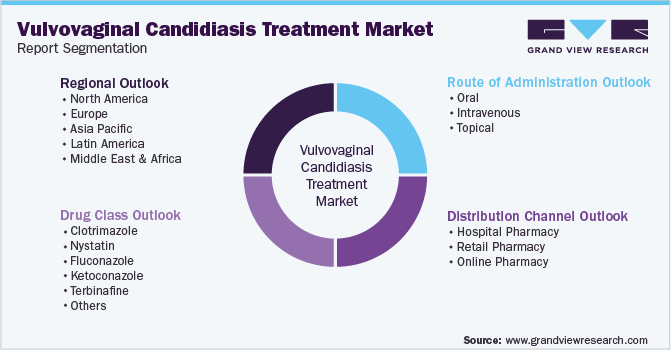
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global vulvovaginal candidiasis treatment market report on the basis of drug class, route of administration, distribution channel, and region:
-
Drug Class Outlook (Revenue, USD Million, 2018 - 2030)
-
Clotrimazole
-
Nystatin
-
Fluconazole
-
Ketoconazole
-
Terbinafine
-
Terconazole
-
Others
-
-
Route of Administration Outlook (Revenue, USD Million, 2018 - 2030)
-
Oral
-
Intravenous
-
Topical
-
-
Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital Pharmacy
-
Retail Pharmacy
-
Online Pharmacy
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
-
Asia Pacific
-
India
-
China
-
Japan
-
Australia
-
South Korea
-
-
Latin America
-
Argentina
-
Brazil
-
Mexico
-
-
Middle East & Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
-
Frequently Asked Questions About This Report
b. The global vulvovaginal candidiasis treatment market size was valued at USD 986.5 million in 2021 and is anticipated to reach USD 1,162.5 million in 2022.
b. The global vulvovaginal candidiasis treatment market is expected to grow at a compound annual growth rate of 4.39% from 2022 to 2030 to reach USD 1.64 billion by 2030.
b. Based on drug class, the fluconazole segment accounted for a share of 45.74% in 2021 due to high prescription of the drugs owing the higher efficacy.
b. Some of the key players in the vulvovaginal candidiasis treatment market are Mycovia Pharmaceuticals, Inc.; Grupo Ferrer Internacional S.A.; Pfizer, Inc.; Bayer AG.; Scynexis, Inc.; Cadila Pharmaceuticals; Bristol-Myers Squibb Company; Basilea Pharmaceutica Ltd.
b. The major factors driving the market growth are the presence of strong product pipeline and increasing incidence of vulvovaginal candidiasis.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





