- Home
- »
- Market Trend Reports
- »
-
Improving Patient Care With Advanced Sterile Processing In 2025 And Beyond
Executive Summary
As healthcare continues to evolve, companies are developing new methods to ensure the highest level of sterilization for medical instruments. Advancements in technology are expanding what's possible, allowing for more efficient, reliable, and innovative solutions to meet the growing demands of sterile processing departments (SPDs). This report discusses product trends, company strategies, statements from key opinion leaders (KOLs), and insights from analysts.

Introduction
The fundamental principle remains constant: thoroughly cleaned, disinfected, and sterilized medical instruments are paramount to preventing infections and ensuring optimal patient outcomes. However, the complexity of modern healthcare, with its increasingly sophisticated surgical procedures and instruments, escalating surgical volumes, and persistent threats of antimicrobial resistance, has propelled sterile processing into a new era of innovation and strategic importance. This report aims to provide a comprehensive overview of the current and emerging trends that are transforming sterile processing, underscoring its pivotal role in enhancing patient safety and satisfaction.
Below Table Represents National standardized infection ratios (SIRs) and facility-specific summary SIRs using adult surgical site infection (SSI) data1 reported to NHSN from NHSN Acute Care
Surgical Procedure
No. of Acute Care
No. of
No. of Infections
Hospitals Reporting2
Procedures
Observed
Predicted3
U.S., all NHSN procedures
3,270
2,971,381
23,938
24,811.222
U.S., SCIP procedures only5
3,239
1,602,268
15,410
16,605.919
AAA Abdominal aortic aneurysm repair5
152
610
5
4.148
AMP Limb amputation
233
17,858
216
72.628
APPY Appendix surgery
497
42,117
115
169.932
AVSD Shunt for dialysis
124
1,368
3
3.557
BILI Bile duct, liver or pancreatic surgery
370
10,868
371
381.145
BRST Breast surgery
358
24,831
204
296.131
CABG- Coronary artery bypass graft5,6
654
115,621
715
950.846
CARD Cardiac surgery5
405
50,864
174
223.494
CEA Carotid endarterectomy
194
6,021
12
5.055
CHOL Gallbladder surgery
511
78,078
339
322.610
COLO Colon surgery5
3,050
331,774
7,789
8,858.806
CRAN Craniotomy
250
48,012
602
541.454
CSEC Cesarean section
673
355,094
877
741.294
FUSN Spinal fusion
928
248,686
2,362
2,016.520
FX Open reduction of fracture
506
67,980
532
502.806
GAST Gastric surgery
468
39,803
195
292.719
HER Herniorrhaphy
306
22,963
148
181.323
HPRO Hip arthroplasty5
2,165
366,097
2,695
2,608.633
HTP Heart transplant
34
1,201
18
16.695
HYST Abdominal hysterectomy5
2,767
250,781
1,846
1,790.612
KPRO Knee arthroplasty5
2,081
460,370
1,905
1,742.560
KTP Kidney transplant
49
6,950
68
41.325
LAM Laminectomy
676
154,988
459
591.169
LTP Liver transplant
36
2,873
80
143.889
NECK surgery
118
3,928
46
82.345
NEPH Kidney surgery
325
12,604
48
41.593
OVRY Ovarian surgery
438
22,324
21
16.937
PACE Pacemaker surgery
326
23,428
52
40.889
PRST Prostate surgery
140
5,415
52
14.079
PVBY Peripheral vascular bypass surgery5
246
8,867
172
178.149
REC Rectal surgery5
431
11,860
78
215.498
SB Small bowel surgery
493
48,003
903
981.069
SPLE Spleen surgery
281
3,054
18
18.411
THOR Thoracic surgery
407
36,796
160
160.081
THYR Thyroid and/or parathyroid surgery
157
4,996
5
3.997
VHYS Vaginal hysterectomy5
493
5,424
31
33.174
VSHN Ventricular shunt
158
5,489
100
78.046
XLAP Abdominal surgery
496
73,385
522
447.605
Source: CDC
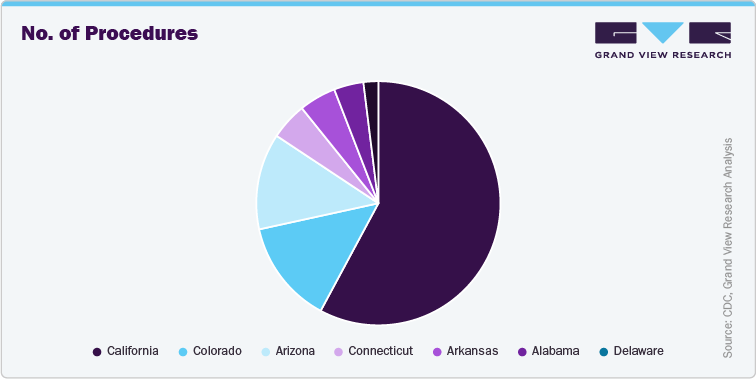
Below Graph represent: State-specific standardized infection ratios (SIRs) and facility-specific SIR summary measures: NHSN Acute Care Hospitals reporting during 2023, Central line-associated bloodstream infections (CLABSI), all locations

Below Graphs represent: State-specific standardized infection ratios (SIRs) and facility-specific SIR summary measures, NHSN Acute Care Hospitals reporting during 2023, Surgical site infections (SSI) following hip arthroplasty1 in adults, ≥ 18years:
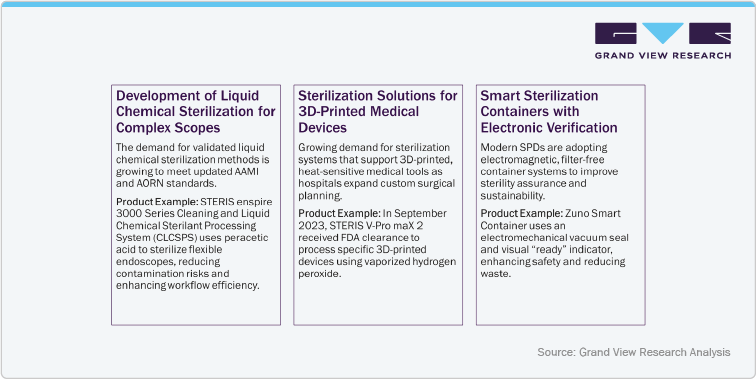
Key Trends and Impact
Technological Advancements in Sterilization and Instrument Reprocessing:
The instruments themselves are becoming more complex, demanding sophisticated reprocessing techniques. This has spurred innovation in sterilization technology.
-
Adoption of HLD: The focus is on high-level disinfection (HLD) and advancements in sterilization technologies for intricate instruments like flexible endoscopes and robotic surgical tools. Innovations include UV-C radiation systems for rapid disinfection without chemicals, and vaporized hydrogen peroxide technology for heat-sensitive and 3D-printed devices. These technologies ensure a higher degree of sterility, reducing the risk of pathogen transmission, especially for devices with complex lumens or materials. Enhanced electronic traceability within these systems provides auditable records, further bolstering safety and compliance.
-
Growing focus on automation of reprocessing: Industry players are launching automated medical device reprocessing solutions to capitalize on the growing demand.. For instance, CS Medical launched its Ethos Automated Ultrasound Probe Cleaner Disinfector, a Class II medical device approved by the U.S. FDA in June 2023. Ethos is the first device in North America to offer both high-level disinfection and cleaning for surface and endocavity ultrasound probes. It reduces the time and labor involved in reprocessing by eliminating manual steps and creating repeatable & validated processes for medical device reprocessing.
Companies are also launching products to protect patients and ensure sterility across modalities. For instance, in October 2023, ASP reported a substantial expansion in its sterilization monitoring portfolio with new steam monitoring products to help SPDs in healthcare facilities ensure greater sterility, efficiency, and effectiveness.
The new solutions support compliance across all major sterilization modalities and include:
-
BIOTRACE Readers - Enable faster result reading and better workflow integration.
-
BIOTRACE Biological Indicators - Offer streamlined, traceable monitoring for steam sterilization.
-
VERISURE Chemical Indicators - Allow quick, easy sterilization process checks.
-
SEALSURE Steam Indicator Tape - Visually distinguishes processed from unprocessed items.
“With a strong history of excellence in Sterile Processing, ASP is extending its expertise into steam sterilization monitoring. This enhanced sterilization monitoring portfolio is aimed at supporting our customers in SPD/CSSD and will allow hospitals to reprocess instruments across all major modalities with greater speed, compliance, and confidence,” said Chad Rohrer, President of ASP. “With this enhanced portfolio, ASP further demonstrates its commitment to advancing sterility assurance and continues its mission to protect patients during their most critical moments.”
Rise of VH₂O₂ Technology in Low-Temperature Sterilization
Healthcare facilities are increasingly adopting Vaporized Hydrogen Peroxide (VH₂O₂) sterilization to process delicate, heat-sensitive instruments safely. This low-temperature, residue-free method ensures effective microbial inactivation while preserving device integrity. The trend reflects growing demand for safer, efficient sterilization and improved infection control in Sterile Processing Departments. Companies are launching VH₂O₂ Technology based products.
For example: in June 2024, Getinge introduced the Poladus 150, an advanced VH₂O₂ low-temperature sterilizer designed for modern surgical needs.
Product & Technology Highlights:
-
VH₂O₂ Sterilization Technology: Delivers efficient, low-residue sterilization suitable for sensitive instruments.
-
Operates at Up to 55°C: Ideal for sterilizing heat- and moisture-sensitive devices.
-
Supports Multiple Instrument Types: Compatible with non-lumen, flexible lumen, and rigid lumen instruments.
-
Cross-Contamination Barrier Technology: Enhances patient safety by minimizing the risk of healthcare-associated infections (HCAIs).
"Today marks a significant milestone for our Infection Control business. The Poladus 150 materializes our ambition to be a leading player in the field of VH2O2 low-temperature sterilization, which is one of the most dynamic segments, with the development of more and more advanced endoscopic and robotic procedures. This product launch is a great addition to our portfolio for central sterile supply departments (CSSDs)," says Stéphane Le Roy, President of Surgical Workflows at Getinge.
“It offers competitive sterilization claims and capacity, ensuring a safe and effective way of reprocessing both flexible and rigid instruments used in endoscopy and surgical operations,” says Sophia Brüggemann, Product Manager at Getinge.
Impact on Patient Safety Due to Technological Advancements
-
Reduces healthcare-associated infections (HCAIs) through effective VH₂O₂ sterilization and HLD.
-
Enables safe sterilization of heat-sensitive and complex surgical instruments.
-
Enhances consistency and reliability in reprocessing with automated systems.
-
Minimizes human error, improving sterility assurance.
-
Supports compliance with infection control standards and protocols.
Workforce Challenges in Sterile Processing Departments
Despite technological advancements, a critical barrier to effective sterile processing remains the shortage of trained personnel and limited awareness around proper reprocessing practices.
-
According to the U.S. Bureau of Labor Statistics, employment for sterile processing technicians is projected to grow by 6% between 2021 and 2031, reflecting increasing demand amid persistent staffing shortages.
These challenges can compromise sterility standards, ultimately affecting patient safety and satisfaction. Addressing this workforce gap is essential to sustain high-quality care and infection prevention in 2025 and beyond.
Some of the key players operating in the sterile processing sector include:

Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


