- Home
- »
- Market Trend Reports
- »
-
Influenza A & B Diagnostic Testing: Emerging Technologies And Market Dynamics
Report Overview
Influenza A and B continue to impose a significant global health and economic burden each year, driving the need for fast, accurate, and accessible diagnostic solutions. The diagnostics landscape for influenza has undergone a profound transformation in recent years, evolving from conventional antigen-based rapid tests toward high-sensitivity molecular and next-generation point-of-care (POC) platforms. This shift reflects both technological innovation and the increasing clinical demand for timely, evidence-based decision-making in respiratory infection management.
Historically, the influenza testing ecosystem has been dominated by rapid antigen detection tests (RIDTs) due to their low cost and quick turnaround time. However, their limited sensitivity particularly during early infection or low viral load phases has constrained their clinical reliability. In contrast, molecular diagnostic platforms, particularly rapid PCR and isothermal amplification-based assays, have gained momentum for delivering laboratory-grade accuracy at or near the point of care.
The market is witnessing a distinct move toward sample-to-answer cartridge systems, which can deliver results within 15–30 minutes without requiring complex instrumentation or technical expertise. These systems are increasingly being adopted in emergency departments, urgent-care settings, and physician offices, where rapid diagnosis directly influences clinical management, infection control, and antiviral prescription decisions.
Influenza A & B Diagnostic Testing: Emerging Technologies and Market Dynamics Report Scope
Attributes
Details
Areas of Research
Industry trends, market opportunity, ease of doing business across countries, competitive analysis
Report Representation
Consolidated report in PDF format
Country Coverage
20+ Countries
Highlights of Report (Competitive Landscape, by country)
- Market Dynamics and Adoption Patterns
- Key Growth Enablers
- Clinical and Economic Benefits
- Regional Adoption Insights
- Digital Integration Opportunities
- Regulatory and Reimbursement Trends
- Diagnostic Trends for 2025
- Future Outlook for the Industry
- Five Strategic Pillars
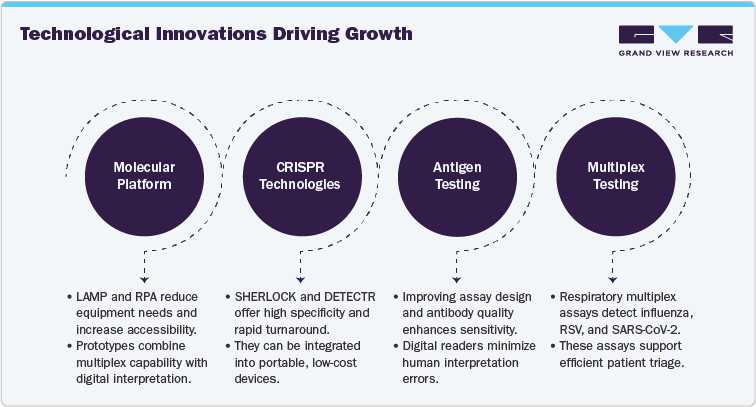
Rapid Molecular and Isothermal Platforms:
Recent developments in loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA) have enabled molecular-level sensitivity without thermocycling, significantly reducing equipment requirements. Integration of these chemistries into microfluidic and paper-based formats has expanded testing accessibility, particularly in decentralized and resource-limited settings. Several prototypes now combine multiplex capability with digital result interpretation, supporting simultaneous detection of Influenza A, Influenza B, and other respiratory pathogens.
CRISPR-Based Diagnostic Technologies:
CRISPR-Cas systems, notably SHERLOCK and DETECTR, are emerging as the next frontier in influenza diagnostics. These assays offer ultra-high specificity, rapid turnaround times, and potential for multiplex detection and strain differentiation. While still in pre-commercial stages, CRISPR-enabled platforms demonstrate strong potential for integration into portable, low-cost devices suitable for both clinical and field applications.
Enhanced Antigen Testing Formats:
Despite their limitations, antigen tests continue to play an important role in high-volume screening and initial triage. Manufacturers are improving assay design and antibody quality to enhance sensitivity, while digital readers are being introduced to minimize human interpretation errors. The trend toward hybrid diagnostic workflows combining antigen screening with reflex molecular confirmation has strengthened overall testing efficiency.
Multiplex and Syndromic Testing Panels:
The post-pandemic diagnostic landscape has accelerated the demand for respiratory multiplex assays capable of detecting influenza, RSV, and SARS-CoV-2 in a single run. Such assays are gaining traction in hospitals and reference laboratories, supporting efficient patient triage and infection control during respiratory virus seasons.
Regional Adoption and Implementation Trends
In the U.S. and Western Europe, rapid molecular diagnostics are now embedded within emergency and outpatient care pathways, with evidence showing improved clinical outcomes, reduced antibiotic prescribing, and shorter emergency department stays. Japan and South Korea remain leaders in POC molecular deployment, supported by early regulatory approvals and strong reimbursement frameworks.
Meanwhile, China and India are witnessing rapid innovation in cost-effective isothermal and paper-based assays driven by domestic manufacturers and public-health initiatives. In Latin America, Africa, and parts of the Middle East, antigen-based tests still dominate, although molecular testing capacity is gradually expanding through donor-funded surveillance programs and private-sector participation.
Clinical and Public-Health Impact
Accurate influenza diagnosis underpins timely antiviral therapy, infection prevention, and rational antibiotic use. Studies have demonstrated that rapid molecular testing contributes to improved antimicrobial stewardship by reducing unnecessary antibiotic prescriptions and enabling faster antiviral initiation. Moreover, molecular and sequencing-capable assays enhance surveillance by enabling subtype characterization and antiviral resistance monitoring critical inputs for vaccine-strain selection and pandemic preparedness.
Beyond individual patient management, diagnostic data integration into digital surveillance systems is emerging as a major public-health enabler. Automated reporting of influenza-positive cases from POC devices to national databases supports near real-time tracking of outbreaks, helping authorities tailor local containment and vaccination strategies.
Emerging Opportunities and Future Outlook
The next phase of innovation will be characterized by greater decentralization, digital integration, and consumer accessibility.
-
At-home and pharmacy-based testing is gaining acceptance following the success of self-administered COVID-19 tests. Manufacturers are now focusing on combining usability with clinical accuracy, ensuring reliable results even in unsupervised settings.
-
Paper-based and microfluidic diagnostic platforms, particularly those utilizing isothermal or CRISPR chemistries, are expected to redefine cost and accessibility paradigms, especially in low-resource regions.
-
Digital connectivity and cloud-based data integration will further enhance the value proposition by linking results to telemedicine platforms, antiviral e-prescriptions, and public-health dashboards.
From a market dynamics perspective, diagnostic manufacturers are diversifying their portfolios to balance clinical and consumer segments. Established players continue to focus on improving throughput and multiplex capabilities, while startups and academic spin-offs are driving disruptive innovation through miniaturized, low-cost molecular and CRISPR-based systems.
Challenges Ahead
Despite promising technological progress, several challenges remain.
-
Regulatory complexity surrounding novel assay formats, particularly CRISPR-based diagnostics, may slow commercialization timelines.
-
Reimbursement variability across markets continues to influence adoption rates, particularly in low- and middle-income countries.
-
Operational barriers, including training requirements and supply chain stability during seasonal peaks, persist in many healthcare systems.
-
Analytical sensitivity and specimen variability remain critical performance determinants, underscoring the need for standardized sampling and clear user instructions.
The influenza diagnostics market is entering a transformative phase, driven by innovation, accessibility, and clinical value creation. Molecular and emerging CRISPR technologies are redefining diagnostic accuracy, while isothermal and paper-based systems promise scalability and affordability. As diagnostic testing becomes increasingly integrated with digital health ecosystems, influenza A and B detection is evolving from a laboratory-dependent process to a real-time, patient-centric, and data-driven ecosystem.
For stakeholders across the healthcare value chain diagnostic developers, providers, payers, and policymakers the focus will now shift from simply detecting influenza to optimizing diagnostic impact through technology convergence, evidence generation, and equitable access.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


