- Home
- »
- Market Trend Reports
- »
-
Molecular Glues Approved Drugs And Pipeline Analysis
Report Overview
Molecular glues are small molecules that facilitate the interaction between a target protein and an E3 ubiquitin ligase, resulting in the ubiquitination and subsequent proteasomal degradation of the target protein. Unlike traditional inhibitors that block the function of proteins, molecular glues eliminate the protein altogether, making them a unique and promising class of drugs in the field of Targeted Protein Degradation (TPD).
One of the key drivers of the molecular glues market growth is the increasing interest in TPD as a novel therapeutic strategy, especially for “undruggable” targets proteins that cannot be modulated effectively by conventional small molecules or biologics. Pharmaceutical and biotech companies are heavily investing in TPD platforms due to their potential to tackle diseases such as cancer, neurodegenerative disorders, and autoimmune conditions. Molecular glues, in particular, offer advantages like simplified chemical structures and oral bioavailability, which contribute to their commercial and clinical appeal.
Despite promising developments, the market faces key restraints such as limited understanding of the molecular glue mechanism and challenges in rational drug design. The discovery of effective molecular glues remains largely serendipitous due to the lack of predictive models for compound-target-ligase interactions. Additionally, safety concerns, off-target protein degradation, and complex regulatory pathways pose hurdles for clinical translation. These scientific and regulatory uncertainties may slow down commercialization timelines, affecting investor confidence and potentially limiting market growth in the short to medium term.
The pharmaceutical industry continually seeks to enhance drug delivery systems to improve treatment efficacy and safety. Molecular glues present a novel approach by enabling the targeted delivery of therapeutics to specific cells or tissues. This precision in delivery can boost drug bioavailability, minimize side effects, and improve overall treatment outcomes. Consequently, the rising demand for more effective drug delivery systems is anticipated to significantly drive the growth of the molecular glues market in the coming years.
Molecular glues offer several advantages over traditional drug delivery methods. They can be engineered to target specific cells or tissues, enhancing treatment efficacy. Additionally, molecular glues can deliver a range of therapeutic agents, such as small molecules, peptides, and proteins. They can also be designed to release their payload gradually, improving patient compliance and reducing the need for frequent dosing. Moreover, the development of next-generation molecular glues with enhanced stability, extended half-life, and more efficient targeting is expected to fuel market growth further, positioning them as an even more promising option for future drug delivery.
Molecular Glue Approved Drugs Analysis Overview
Molecular glue drugs have gained attention for their unique mechanism of promoting targeted protein degradation by enhancing protein–protein interactions. One of the most notable success stories in this space is lenalidomide, a thalidomide analog developed by Celgene (now part of Bristol Myers Squibb). Lenalidomide and its related compounds (e.g., pomalidomide and thalidomide itself) function as molecular glues by promoting the interaction between cereblon (CRBN), an E3 ubiquitin ligase, and specific target proteins such as Ikaros and Aiolos, leading to their degradation. These drugs are approved for the treatment of multiple myeloma and certain myelodysplastic syndromes and have been pivotal in validating the molecular glue concept clinically.
List of FDA-Approved Molecular Glues
International
nameFDA brand
namePPI
MOA
FDA
approved yearThalidomide
Contergan, Thalomid
CRBN/IKAROS-family
Type II/III
1998
Lenalidomide
Revlimid
CRBN/CK1α
Type II
2005
Pomalidomide
Pomalyst
CRBN/IKAROS-family
Type II/III
2013
Source: PatSnap, FDA, Investor Presentations, Primary Interviews, Grand View Research
The success of lenalidomide and its analogs has demonstrated the therapeutic potential of molecular glues, particularly in hematological malignancies. Their clinical utility has not only revolutionized multiple myeloma treatment but also provided a platform for the development of next-generation glues with improved specificity and safety profiles. These approvals underscore the viability of the molecular glue mechanism and have driven increased interest from biotech startups and pharmaceutical companies looking to expand the concept to new therapeutic areas, including solid tumors and neurodegenerative diseases.
Molecular Glues Pipeline Analysis Overview
The molecular glues pipeline is gaining significant momentum, with increasing focus from both biotech startups and major pharmaceutical companies. Initially centered around hematological malignancies, current research and development efforts are expanding into solid tumors, neurodegenerative diseases, inflammatory conditions, and infectious diseases. Companies are leveraging molecular glue technology to address previously "undruggable" targets, particularly transcription factors and scaffold proteins, which cannot be modulated through conventional small molecules. This expansion reflects the growing confidence in molecular glues as a versatile and effective therapeutic modality.
Molecular Glues Pipeline Drugs
Molecular Glues
Company name
Phase
Indication
Mechanism of Action (MoA)
Mezigdomide
Bristol Myers Squibb
Phase III
Multiple myeloma
Apoptosis stimulants; CRBN protein modulators; Ubiquitin protein ligase modulators
Golcadomide
Bristol Myers Squibb
Phase III
Lymphoma
CRBN protein modulators; Ubiquitin protein ligase modulators
Iberdomide
Bristol Myers Squibb
Phase III
Multiple Myeloma
Ubiquitin protein ligase complex modulators
MRT-2359
Monte Rosa Therapeutics, Inc
Phase I/II
Solid tumors
Peptide-chain-release factor 3 degraders
RMC 6291
Revolution Medicines, Inc.
Phase I/II
Solid tumors
KRAS protein inhibitors
NX-2127
Nurix Therapeutics
Phase I
B-cell malignancies
Agammaglobulinaemia tyrosine kinase degraders; Agammaglobulinaemia tyrosine kinase inhibitors; IKZF1 protein degraders; IKZF3 protein degraders
NST-628
Nested Therapeutics
Phase I
Solid tumors
Mitogen-activated protein kinase inhibitors; Ras protein modulators
SP-3164
DeuteRx
Phase I
Non-Hodgkin's Lymphoma
CRBN protein modulators; IKZF1 protein degraders; IKZF3 protein degraders
Source: FDA, Investor Presentations, Primary Interviews, Grand View Research
Several prominent players are driving innovation in this space, including Bristol Myers Squibb, C4 Therapeutics, Arvinas, Monte Rosa Therapeutics, and Amphista Therapeutics. These companies are actively developing next-generation molecular glues that engage various E3 ligases beyond the commonly used CRBN (cereblon). Strategic collaborations and licensing agreements between large pharmaceutical companies and specialized biotech firms are fueling pipeline growth. For instance, Bristol Myers Squibb’s partnership with Evotec and C4 Therapeutics exemplifies how big pharma is investing in external innovation to diversify its molecular glue pipeline.
While most candidates in the molecular glues pipeline are in preclinical or early-phase clinical development, several have entered Phase I and II trials, signaling a transition toward clinical validation. Technological advances in structural biology, proteomics, and ligand-based drug discovery are accelerating the identification of viable glue compounds. Furthermore, the advancement of novel screening platforms enabling the systematic identification of molecular glue candidates, moving beyond chance-based methods, is instrumental in broadening the pipeline. As these tools mature and more candidates advance through clinical stages, the molecular glues pipeline is poised to become a major pillar in the targeted therapy landscape.
Molecular Glues in Clinical Trials
Name
Clinical trial
Phase
Company
Target
Indications
CC-92480
NCT05372354
Phase II
BMS/Celgene
IKZF1/3
RRMM
CC-220
NCT02773030
Phase II
BMS/Celgene
IKZF1/3
MM
E7820
NCT05024994
Phase II
Eisai
RBM39
AML, MDS
ICP-490
NCT05719701
Phase II
InnoCare
IKZF1/3
RRMM
MRT-2359
NCT05546268
Phase I/II
Monte Rosa
GSPT1
SCLC, NSCLC, DLBCL
CFT-7455
NCT04756726
Phase I/II
C4 Therapeutics
IKZF1/3
RRMM
DKY-709
NCT03891953
Phase I/Ib
Novartis
IKZF2
NSCLC, melanoma
CC-90009
NCT04336982
Phase I
BMS/Celgene
GSPT1
AML
Source: drugdiscoverytoday, FDA, Investor Presentations, Primary Interviews, Grand View Research
Molecular Glues Application Analysis Overview
One of the most prominent applications of molecular glues is in oncology, particularly in hematologic malignancies such as multiple myeloma and certain types of lymphomas. Approved drugs like lenalidomide and pomalidomide are already being used to treat tens of thousands of patients globally, with multiple myeloma alone affecting over 160,000 people worldwide annually. These molecular glues work by targeting transcription factors like Ikaros and Aiolos for degradation, which are key drivers of cancer cell survival and proliferation. As research continues, molecular glues are being investigated for broader oncology applications, including solid tumors such as glioblastoma and lung cancer, where conventional treatments are limited or ineffective.
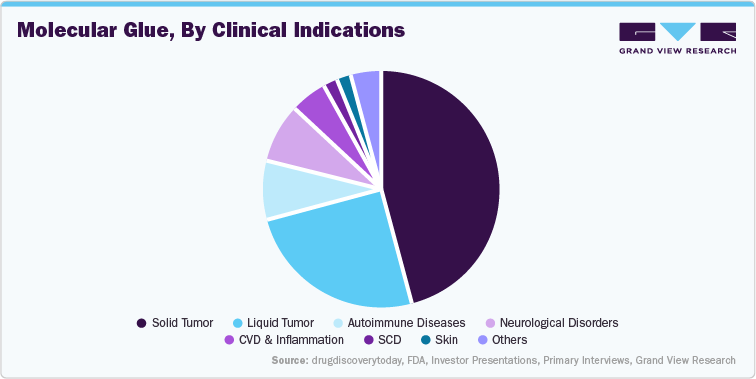
Molecular glues are also emerging as potential treatments for neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and Huntington’s disease. These conditions, which collectively affect hundreds of millions of people globally, are characterized by the accumulation of toxic proteins in the brain. Molecular glues offer a novel strategy by promoting the degradation of such pathogenic proteins, like tau and alpha-synuclein. Although still in the preclinical stage, the ability of molecular glues to selectively target and degrade disease-driving proteins without affecting normal cellular function could transform how neurodegenerative diseases are managed, offering hope to a large, aging patient population with few effective therapies.
Another promising area for molecular glue application is in rare and genetic diseases, where many conditions are driven by specific protein mutations or dysregulated protein activity. Disorders such as spinal muscular atrophy (SMA), Duchenne muscular dystrophy (DMD), and certain inherited metabolic conditions are potential targets. While the individual patient populations for these diseases are small, often ranging from a few thousand to a few hundred thousand globally, the high unmet need and orphan drug incentives make these applications attractive from both a clinical and commercial standpoint. Molecular glues could provide a targeted and potentially curative approach in these settings, especially by degrading mutant or misfolded proteins central to disease pathology.
Key Takeaways From The Molecular Glues Competitive Landscape
Leading companies in the molecular glues sector, such as Bristol Myers Squibb; Monte Rosa Therapeutics Inc.; Revolution Medicines Inc.; Nurix Therapeutics; Nested Therapeutics; and DeuteRx, are actively exploring new molecular glues to enhance the treatment landscape.
-
In May 2024, Takeda Pharmaceuticals entered into an exclusive licensing agreement with Degron Therapeutics, a China-based company, to develop novel molecular glue degraders targeting various oncology, neuroscience, and inflammatory disease indications.
-
In May 2024, NEOsphere Biotechnologies GmbH announced a collaboration with Kymera Therapeutics, Inc. to target disease-causing proteins that are difficult to address with traditional therapies. Through this partnership, NEOsphere will leverage its target- and E3-agnostic platform to screen molecular glue compounds on a proteome-wide scale in native contexts.
-
In March 2024, Nurix Therapeutics, Inc. revealed that the FDA had lifted the partial clinical hold on its U.S. Phase Ia/Ib study for NX-2127 in adults with relapsed/refractory B-cell malignancies. The hold, initially announced in November 2023, was due to the company’s plan to transition to an improved manufacturing process.
-
In November 2023, Kymera Therapeutics reported promising results from the Phase I clinical trial of its lead program, KT-474, marking a significant milestone in the targeted protein degradation field.
-
In September 2023, Orionis Biosciences announced a multi-year collaboration with Genentech, a member of the Roche Group, aimed at discovering novel small-molecule drugs for challenging targets in oncology and neurodegeneration. Under the agreement, Orionis will focus on discovering and optimizing molecular glues for Genentech’s designated targets, while Genentech will manage later-stage preclinical, clinical development, regulatory filings, and commercialization.
Big Pharma Partnership Deals With Start-up Firms Developing Molecular Glues
Start-up firm
Pharmaceutical company
Year
Upfront money (deal amount)
Neomorph
Novo Nordisk
2024
Eligible for $1.46 billion
VantAI
BMS
2024
Eligible for $674 million
Proxygen
Merck
2023
Eligible for $2.55 billion
Orionis
Genentech
2023
$47 million (eligible for $2 billion)
Source: drugdiscoverytoday, FDA, Investor Presentations, Primary Interviews, Grand View Research
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


