- Home
- »
- Market Trend Reports
- »
-
Secukinumab (Cosentyx): Navigating The Patent Cliff and Strategic Implications
Report Overview
Secukinumab (Cosentyx), developed by Novartis, is a leading IL-17A inhibitor approved for multiple immune-mediated conditions, including plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, and hidradenitis suppurativa, making it a cornerstone of Novartis’s immunology portfolio. In 2024, Cosentyx generated around USD 6.14 billion in net sales, reflecting strong market adoption across major regions. The core U.S. patent is expected to expire by 2029, while European exclusivity extends until 2030, with secondary patents potentially prolonging protection into the early 2030s. As this patent cliff approaches, Cosentyx is likely to face biosimilar competition and pricing pressure, though Novartis is actively mitigating risks through lifecycle management strategies, including label expansions, new indications, pediatric approvals, and real-world evidence generation. Despite upcoming competitive challenges, Cosentyx is expected to remain a significant revenue driver for Novartis through the late 2020s, with long-term market performance influenced by biosimilar adoption rates and strategic brand differentiation.
Key Report Deliverables
-
Detailed analysis of the Cosentyx market landscape, covering revenue size, immunology growth drivers, global adoption trends, and the evolving competitive context shaping the IL-17 inhibitor and broader autoimmune therapeutic industry.
-
Forecasts evaluating post-patent market growth trajectories, expected biosimilar entry timelines across regions, and the projected impact on revenue streams, pricing dynamics, and market access in psoriasis, psoriatic arthritis, ankylosing spondylitis, and other approved indications.
-
Identification of regulatory and market barriers affecting biosimilar penetration, including approval complexities, interchangeability requirements, pricing policies, and payer-driven access restrictions across different geographies worldwide.
-
Comprehensive competitive landscape overview, highlighting direct IL-17-targeting therapy rivals (e.g., Ixekizumab, Bimekizumab), emerging pipeline candidates, next-generation immunology therapies, and innovation strategies shaping the autoimmune treatment ecosystem.
-
Strategic implications for Novartis and competitors, focusing on lifecycle management, new formulations, expanded indications, innovation pipelines, pricing strategies, and regional positioning to sustain leadership post-patent.
Current Market Scenarios
Cosentyx continues to benefit from strong patent protection and regulatory exclusivities across major global markets, though expiry timelines vary by region. In the U.S., the core patent is expected to expire around 2029, creating a potential window for biosimilar entry. In Europe, patents and supplementary protection certificates could extend exclusivity until 2030, giving Novartis a longer period of market defense. Japan and Canada follow similar timelines, whereas China and India may face earlier exposure to biosimilar competition due to local patent regulations and faster regulatory approvals for biosimilars. This uneven schedule is likely to drive region-specific competitive and pricing dynamics.
The U.S., representing Cosentyx’s largest revenue market, could see price erosion and shifts in market share once biosimilars launch. Europe, with cost-conscious health systems, is expected to adopt biosimilars relatively quickly, intensifying downward pricing pressure. In China and India, domestic biosimilar developers are advancing rapidly, supported by government initiatives to enhance treatment accessibility. Japan, despite its high market value, may experience slower biosimilar uptake due to stringent regulatory requirements and gradual clinical adoption, creating uneven adoption patterns across Asia-Pacific.
Despite these challenges, Cosentyx demand remains robust, supported by expanded indications, earlier intervention strategies, real-world evidence, and innovation, including new formulations, positioning the therapy to maintain a leading role in the global immunology market.

Market Dynamics
Growing Demand for IL-17-Targeting Therapies
The global rise in autoimmune and inflammatory disorders, particularly plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis, is driving strong demand for Cosentyx. As a leading IL-17A inhibitor, it has become a standard of care across multiple indications, supported by robust clinical efficacy and safety profiles. Broad adoption, label expansions, and updated treatment guidelines reinforce Cosentyx’s central role in immunology therapy, sustaining strong demand worldwide.
Pricing and Market Erosion Post-Patent
With the anticipated patent expiry in 2029 (U.S.) and 2030 (EU), Cosentyx is expected to face biosimilar competition, introducing downward pricing pressure. While biosimilars may initially encounter clinical caution, payers and governments are likely to encourage adoption of cost-effective alternatives. This is expected to accelerate uptake in Europe, China, and India, reshaping competitive dynamics and eroding Novartis’s market exclusivity.
Opportunities in Lifecycle Management
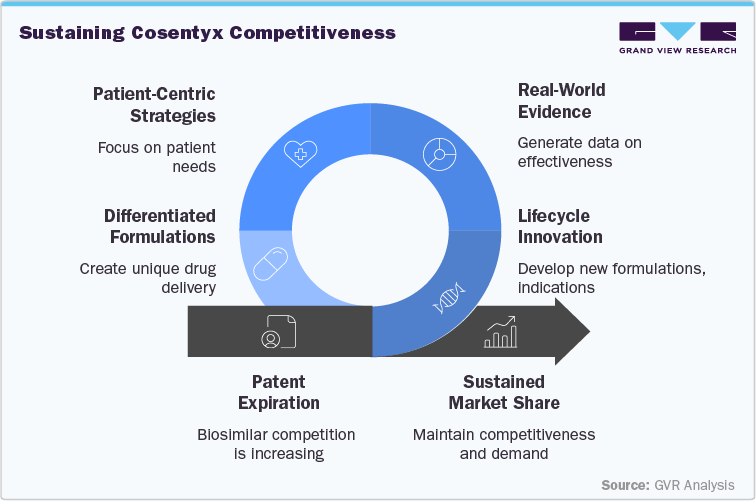
Novartis is actively defending Cosentyx’s market position through expanded indications, pediatric approvals, and new formulations. Beyond lifecycle extensions, the company is investing in next-generation immunology therapies, combination regimens, and real-world evidence to maintain differentiation. These strategies aim to sustain revenue streams and strengthen long-term leadership despite increasing biosimilar competition.
Regional Impacts Post-Patent
Post-patent effects are expected to vary by region. Mature markets like the U.S., EU, and Japan may experience slower biosimilar adoption due to strict regulations and cautious clinical uptake, whereas emerging markets such as China, India, and Latin America are likely to transition faster, driven by affordability initiatives, government incentives, and cost-focused healthcare policies.
The Pressure of Pricing and Market Erosion Post-Patent
The expiration of Cosentyx patents is expected to trigger increased competition from biosimilar IL-17 inhibitors, significantly impacting pricing dynamics and market share across approved autoimmune indications. In the post-patent environment, downward pricing pressure is inevitable, particularly in cost-sensitive markets where payers prioritize affordable immunology care. As lower-cost alternatives enter, Novartis’s market share may erode, with physicians and patients considering biosimilar options. However, biosimilars often face initial adoption barriers due to clinical comparability concerns and efficacy confidence, especially in chronic conditions like psoriasis and psoriatic arthritis.
The regulatory approval process will influence the pace of biosimilar penetration. In mature markets such as the U.S., EU, and Japan, stringent requirements may delay entry, whereas China, India, and Latin America are likely to see earlier launches supported by domestic manufacturers and streamlined pathways. While physicians may initially be cautious about switching patients purely on cost, payer policies, tenders, and reimbursement incentives will accelerate adoption over time. Ultimately, biosimilar entry will reshape the competitive landscape, compelling Novartis and rivals to focus on lifecycle management, innovative formulations, expanded indications, and patient-centric strategies to sustain leadership post-patent.

Innovating Beyond the Patent - Unlocking Future Growth Paths
Despite the inevitable biosimilar competition following Cosentyx’s loss of exclusivity, the autoimmune therapy market is expected to continue offering meaningful growth opportunities. A key avenue lies in the development of next-generation formulations, including new delivery methods that enhance convenience, improve adherence, and expand patient adoption. Additionally, ongoing research into combination regimens-pairing IL-17 inhibition with other targeted therapies or novel biologics-offers differentiated positioning and reinforces Cosentyx’s role in multi-modal immunology care.
Innovation will also be critical to maintaining clinical relevance amid pricing pressures. Novartis is advancing new immunology assets, exploring novel formulations and expanded indications, which can extend leadership beyond the current IL-17 inhibitor portfolio. The rise of biosimilars may also create market access opportunities in emerging economies, where affordability has historically limited uptake. Strategic partnerships or authorized biosimilar programs could expand treatment access while preserving brand presence.
Another growth driver lies in the expansion into emerging markets, where autoimmune disease prevalence is rising and healthcare infrastructure is improving. Countries across Asia, Latin America, and Africa are witnessing increased demand for effective therapies. By ensuring the availability of both branded and cost-effective alternatives, Novartis can broaden patient access and sustain Cosentyx’s strategic relevance in the global immunology market.
Shaping the Future - Biosimilars, Patient-Centric Models, and Regional Shifts
-
Shift Towards Patient-Centric Immunology Treatments
There is a growing emphasis on patient-centric autoimmune therapies, focusing on convenience, quality of life, and long-term disease management. Innovations such as new Cosentyx formulations, self-injectable options, and tailored dosing regimens enhance therapy personalization. These approaches address multiple patient needs simultaneously, reinforcing the relevance of IL-17-targeting therapies in delivering comprehensive immunology care, even in the face of biosimilar competition.
-
Adoption of Value-Based Healthcare Models
The global transition toward value-based healthcare is accelerating, with cost-effectiveness and measurable patient outcomes guiding treatment decisions. This trend will encourage the uptake of IL-17 biosimilars, particularly in price-sensitive markets where healthcare budgets are constrained. Payers and providers will increasingly prioritize therapies that maintain clinical efficacy while offering cost savings, reshaping competitive dynamics for Cosentyx and emerging biosimilar challengers.
-
Regional Divergence in Competitive Dynamics
Following Cosentyx’s patent expiry, regional variations will strongly influence adoption trends. In mature markets such as the U.S., EU, and Japan, biosimilar uptake may advance slowly due to regulatory caution and prescriber hesitancy. In contrast, emerging markets like China, India, Brazil, and Latin America are likely to transition faster, driven by affordability pressures, supportive government policies, and streamlined biosimilar approval pathways.
Overview of Alternative Therapeutics
Cosentyx faces direct competition from other IL-17-targeting therapies such as Ixekizumab (Eli Lilly) and Bimekizumab (UCB), which are expanding indications, dosing regimens, and geographic presence to capture larger shares of the autoimmune therapy market. Beyond IL-17 inhibitors, emerging innovations including IL-23 inhibitors, JAK inhibitors, and next-generation monoclonal antibodies are shaping the future treatment landscape, increasing competitive pressures on Cosentyx.
A wave of Cosentyx biosimilars is progressing through global clinical programs, led by manufacturers including Celltrion and other regional developers. These entrants are preparing for market launches following key patent expirations in the U.S. (2029) and EU (2030). Their success will depend on demonstrating clinical comparability, obtaining regulatory approvals, achieving cost advantages, and executing pricing strategies to penetrate autoimmune markets, where affordability and payer acceptance strongly influence adoption and overall market dynamics.
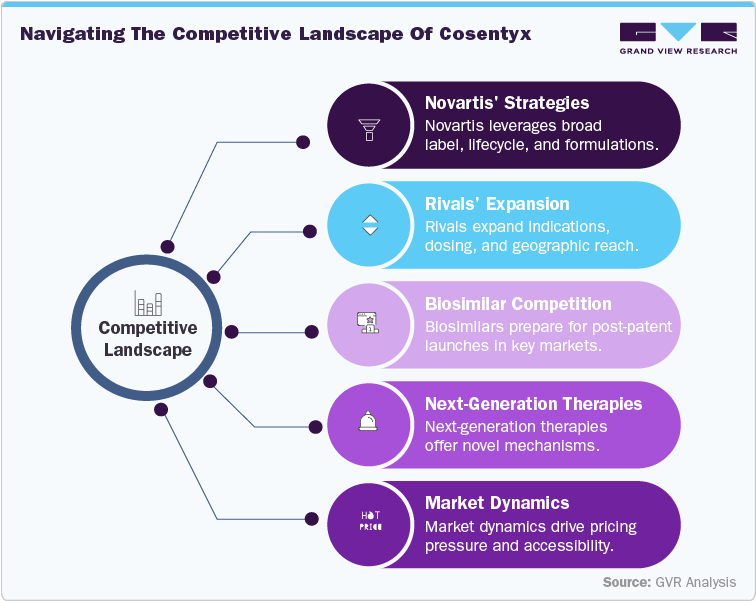
Competitive Landscape
The competitive landscape for Cosentyx is evolving rapidly, with key players pursuing diverse strategies to sustain leadership as the patent cliff approaches. Novartis, the dominant player, continues to leverage Cosentyx’s broad label portfolio, lifecycle management initiatives, and development of next-generation formulations to maintain market share. Meanwhile, rivals such as Ixekizumab (Eli Lilly) and Bimekizumab (UCB) are aggressively expanding indications, dosing regimens, and geographic reach, intensifying competition across the autoimmune therapy market.
Indirect competition from biosimilars is progressing quickly. Companies including Celltrion and other regional developers are conducting clinical programs to prepare for post-patent launches in the U.S. (2029) and EU (2030). China-based innovators are strengthening local competition, supported by government policies that accelerate biosimilar adoption. Additionally, emerging next-generation therapies, including IL-23 inhibitors, JAK inhibitors, and novel monoclonal antibodies, represent long-term competitive pressures due to promising efficacy and novel mechanisms of action.
To safeguard their positions, Novartis is emphasizing lifecycle extensions, global collaborations, patient-support programs, and real-world evidence generation. Competitors are focusing on pipeline diversification, expanded indications, and digital health engagement to reinforce adoption. The entry of biosimilars and next-generation therapies, especially in emerging markets, is expected to drive pricing pressure, broaden accessibility, and reshape treatment paradigms. Ultimately, companies’ ability to innovate, differentiate, and deliver patient-centric solutions will determine resilience in navigating the post-patent autoimmune therapy landscape.
North America Secukinumab (Cosentyx) Market
North America remains the largest market for Cosentyx, led primarily by the U.S., which accounts for the majority of Novartis’s IL-17 inhibitor revenues. The core U.S. patent is expected to expire in 2029, creating opportunities for Cosentyx biosimilars to enter and influence pricing. The U.S. market is likely to experience significant shifts post-expiry, with payers favoring cost-effective therapies. In Canada, where exclusivity also ends in the late 2020s, biosimilars may launch sooner, intensifying competition. While regulatory and clinical adoption hurdles could delay immediate penetration, substantial price erosion is expected once biosimilars gain payer and prescriber confidence.
Europe Secukinumab (Cosentyx) Market
The European Cosentyx market is robust, with Germany, France, and the U.K. being the largest contributors. Patent protections, supported by Supplementary Protection Certificates (SPCs), extend exclusivity until 2030, giving Novartis a slightly longer runway compared to North America. Once biosimilars gain approval, Europe’s cost-sensitive healthcare systems are expected to prioritize lower-cost alternatives. Stringent regulatory requirements may slow initial adoption, but tender-based procurement and competitive pricing pressures will rapidly reshape market dynamics in favor of biosimilars.
Asia Pacific Secukinumab (Cosentyx) Market
The Asia Pacific region presents significant growth opportunities, particularly in China, India, and Japan, where autoimmune disease awareness and treatment uptake are rising. China may see earlier biosimilar entry due to local patent regulations and strong government support, intensifying competition. In India, affordability is a key driver, and biosimilars are expected to gain rapid traction once exclusivity lapses. Japan, with its strict regulatory framework, may experience slower biosimilar adoption despite high demand for advanced therapies. Overall, regulatory timelines and local competition will strongly influence Cosentyx’s market trajectory.
Latin America Secukinumab (Cosentyx) Market
In Latin America, increasing prevalence of autoimmune conditions drives demand for Cosentyx. Key markets include Brazil, Mexico, and Argentina, although affordability challenges remain central to adoption. Following patent expiry, wider biosimilar availability is expected, improving access and reducing costs. Regulatory agencies in Brazil and Mexico may facilitate faster biosimilar approvals, accelerating competitive dynamics, but logistical barriers and infrastructure limitations could restrict penetration in certain areas, making pricing and distribution strategies critical.
Middle East and Africa Secukinumab (Cosentyx) Market
The MEA market for Cosentyx is emerging, with lower penetration relative to developed regions. Key markets include Saudi Arabia, UAE, and South Africa, where demand is rising due to increasing awareness of autoimmune diseases. High branded therapy costs limit access, making the introduction of biosimilars post-patent expiry essential for expansion. Regulatory pathways vary, with the UAE and Saudi Arabia offering relatively efficient approvals, while other countries may face delays. Nevertheless, improving healthcare infrastructure and patient access are expected to drive long-term growth.
Analyst Perspective
The Cosentyx market is approaching a pivotal transition as its core patents near expiration, beginning in the U.S. in 2029 and in Europe by 2030. Currently dominated by Novartis, the market will face increasing competition from Cosentyx biosimilars, which are expected to drive price reductions and market share shifts. At the same time, rival IL-17-targeting therapies such as Ixekizumab (Eli Lilly) and Bimekizumab (UCB)-along with next-generation immunology therapies including IL-23 inhibitors, JAK inhibitors, and novel monoclonal antibodies-are set to intensify competitive pressures, particularly in price-sensitive regions.
Despite these challenges, demand for Cosentyx and other IL-17 inhibitors is expected to remain strong, supported by proven efficacy, expanding indications, and continued integration into treatment guidelines. To sustain competitiveness, Novartis and other players must prioritize lifecycle innovation, real-world evidence generation, patient-centric strategies, and differentiated formulations to navigate the evolving autoimmune therapy landscape effectively.
Case Study (Recent Engagement): Keytruda Patent-Cliff & Price- Erosion Impact Model
PROJECT OBJECTIVE
To evaluate the potential revenue, price, and patient access implications of Keytruda’s 2028 patent cliff, incorporating biosimilar entry dynamics, country-specific adoption curves, and Merck’s lifecycle defense strategies (remarkably the subcutaneous formulation). The goal was to provide the client with a transparent, scenario-based model to anticipate outcomes and inform strategy
GVR SOLUTION
-
Built a bottom-up commodity-flow and analogue-based model, anchored on Merck’s $29.5B Keytruda sales in 2024.
-
Integrated jurisdictional LOE timelines (EU mid-2028, U.S. 2028-2029 pending litigation outcomes).
-
Modeled biosimilar adoption S-curves calibrated to oncology antibody analogues (EU faster via tenders, U.S. slower via contracting).
-
Applied price-erosion benchmarks (EU -15-30% Yr-1, deepening to -45-60% by Yr-3; U.S. -10-25% net decline over same horizon).
-
Layered lifecycle defenses (SC uptake assumptions of 25-40% of innovator units, combo refresh, contracting) to quantify buffers.
-
Delivered outputs as a dynamic Excel scenario tool and a management-ready PPT deck with revenue bridges, sensitivity tornadoes, and SC migration visuals.
IMPACT FOR CLIENT
-
Enabled the client to quantify downside vs. defense-optimized revenue trajectories:
-
Base case: 30-40% global revenue decline by Year-3 post-LOE.
-
Downside: 45-55% decline in tender-heavy markets.
-
Defense-optimized: Contained erosion to ~-20-25% with strong SC adoption.
-
-
Gave the client a clear view of which markets drive early erosion (EU) and where strategic contracting or SC migration can preserve share (U.S.).
-
Equipped decision-makers with a playbook of watch-points (tender concentration, litigation outcomes, SC IP coverage, combo pipeline) to guide commercial strategy.
-
Provided a transparent methodology that could be presented to boards/investors with evidence-backed assumptions
WHY THIS MATTERS
-
Keytruda is the world’s best-selling cancer drug, representing nearly one-third of Merck’s revenue.
-
Patent expiry will reshape both Merck’s earnings profile and global oncology access dynamics.
-
Payers and governments stand to benefit from biosimilar entry through lower costs, but manufacturers need to manage cliff risk while capturing upside from lifecycle innovations.
-
Understanding how quickly revenues erode and how patient access expands post-biosimilar is critical for:
-
Biopharma companies (strategic planning, pipeline prioritization).
-
Investors (valuing Merck’s cash flows beyond 2028).
-
Payers and policymakers (budgeting for oncology drug spend).
-
A robust patent cliff model helps clients navigate the dual challenge of price erosion and patient expansion, ensuring strategies are grounded in real-world benchmarks.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


