Acute Repetitive Seizures Market Trends
The global acute repetitive seizures market size was estimated at USD 3.15 billion in 2024 and is projected to grow at a CAGR of 12.7% from 2025 to 2030. According to World Health Organization (WHO), epilepsy is a neurological condition affecting about 50 million people worldwide. Many existing therapies fail to provide adequate control of acute repetitive seizures and may come with undesirable adverse effects. This gap in effective treatment solutions prompts pharmaceutical companies to prioritize innovation and the development of novel therapeutic options that can better serve patient requirements. As healthcare providers increasingly seek reliable treatments, the incentive for pharmaceutical advancements in this area continues to grow.
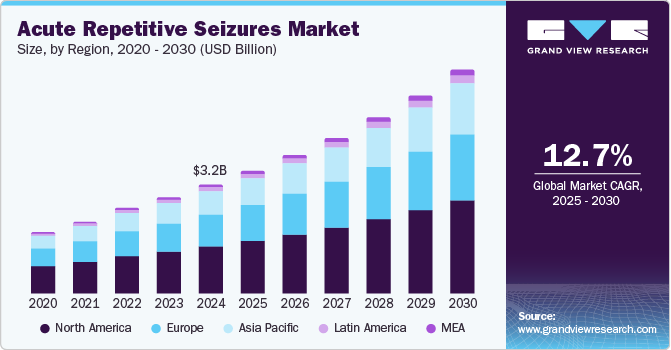
The rising prevalence of neurological disorders, particularly epilepsy, is another significant driver contributing to the growth of the Acute Repetitive Seizures Market. Approximately 50 million people globally are affected by epilepsy, which underscores a substantial patient population in urgent need of effective management strategies. This increasing incidence not only heightens the demand for treatment options but also encourages healthcare stakeholders to invest in therapies that can significantly improve the quality of life for those afflicted by these conditions.
Moreover, advancements in the product pipeline are crucial for market expansion. Ongoing research and development efforts are yielding new drug approvals and innovative treatment modalities, which are essential for enhancing patient outcomes. As more effective therapies become available, the overall demand for treatments targeting acute repetitive seizures is expected to rise, driving further growth in the market. The integration of innovative solutions in clinical settings reinforces the importance of continued investment in research to address existing treatment shortfalls.
An increase in awareness surrounding seizure management and proactive healthcare initiatives are facilitating better diagnosis and treatment options, particularly in emerging markets. The growing trend towards outpatient care-such as home healthcare and telemedicine-further enhances accessibility for patients while reducing hospitalization costs. Combined with increased investment in neurology research, primarily in regions such as North America and Europe, these key factors create a conducive environment for the industry to thrive in the forthcoming years.
Product Insights
USL-261 dominated the market and accounted for a share of 44.5% in 2024, owing to its effectiveness as an intranasal treatment aimed at achieving rapid seizure control. Its user-friendly administration and favorable safety profile position it as the preferred option among healthcare providers, effectively addressing significant unmet clinical needs in the management of acute seizures.
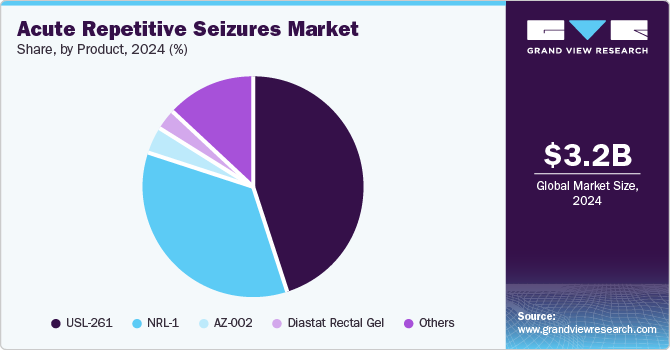
AZ-002 is projected to grow at the fastest CAGR of 36.1% over the forecast period, offering a novel formulation that prioritizes rapid action and enhances patient compliance. Developed as a nasal spray, AZ-002 significantly improves convenience for both patients and caregivers, resulting in its increasing adoption within clinical settings and contributing to an overall rise in treatment efficacy.
Regional Insights
North America acute repetitive seizures market dominated the global market with a revenue share of 43.0% in 2024. Market growth in the region is driven by a high prevalence of epilepsy and neurological disorders, complemented by a robust healthcare infrastructure. Significant investments in research and development, along with a favorable regulatory environment and access to advanced treatment options, reinforce the region's dominance, attracting both patients and pharmaceutical companies.
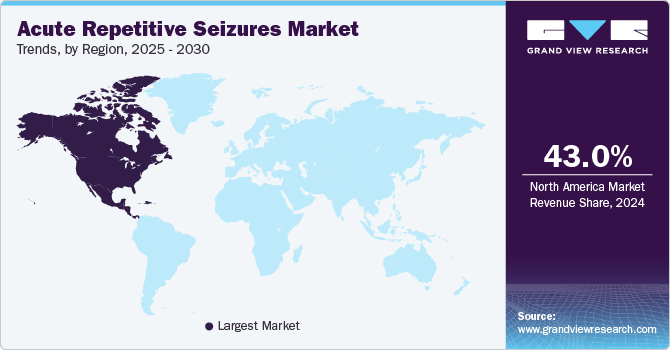
U.S. Acute Repetitive Seizures Market Trends
The acute repetitive seizures market in the U.S. dominated the North America acute repetitive seizures market with the largest revenue share in 2024. Elevated healthcare expenditures, the presence of leading pharmaceutical companies, and ongoing advancements in drug development collectively enhance accessibility to treatments. Furthermore, increased awareness and improved diagnostic capabilities significantly contribute to the market's expansion in the U.S.
Europe Acute Repetitive Seizures Market Trends
Europe acute repetitive seizures market held substantial market share in 2024 due to rising investments in neurology research and an emphasis on innovative treatment solutions. Strong government support for healthcare initiatives, heightened awareness surrounding seizure management, and an increased demand for effective medications facilitate advancement in therapeutic options.
The acute repetitive seizures market in Germany is expected to grow in the forecast period. The nation's commitment to advancing neurology treatments, combined with a high prevalence of epilepsy, fosters strong demand for effective therapies. Germany’s regulatory environment further supports rapid drug approvals, significantly bolstering market growth and innovation.
Asia Pacific Acute Repetitive Seizures Market Trends
Asia Pacific acute repetitive seizures market is expected to register the fastest CAGR of 14.2% in the forecast period, supported by a rising incidence of neurological disorders and increased awareness of epilepsy treatment options. Developing healthcare infrastructure and growing investments in medical research and development position the region for substantial growth as patient-centric care becomes increasingly prioritized.
The acute repetitive seizures market in Japan dominated the Asia Pacific acute repetitive seizures market in 2024. The aging population, coupled with rising awareness of neurological disorders, stimulates demand for effective therapies. Furthermore, Japan’s commitment to research and development significantly enhances its competitive position within the region.
Key Acute Repetitive Seizures Company Insights
Some key companies operating in the market include UCB S.A., Belgium; Neurelis, Inc.; Bausch Health Companies Inc.; and Grupo Ferrer Internacional, S.A.; SERB. Strategic initiatives emphasize significant R&D investments for innovative therapies, partnerships with healthcare providers for improved distribution, and marketing efforts to boost patient awareness and access to treatments.
-
Neurelis, Inc. is a biotechnology firm dedicated to creating pioneering therapies for epilepsy and central nervous system disorders. Their primary offering, VALTOCO®, is an FDA-approved intranasal diazepam spray for managing intermittent seizures in individuals aged six and over.
-
Grupo Ferrer Internacional, S.A., a pharmaceutical company, specializes in developing and marketing a range of healthcare products, particularly for neurological disorders. They emphasize innovative formulations and delivery systems in the industry to enhance treatment efficacy and patient compliance.
Key Acute Repetitive Seizures Companies:
The following are the leading companies in the acute repetitive seizures market. These companies collectively hold the largest market share and dictate industry trends.
- UCB S.A., Belgium
- Neurelis, Inc.
- Bausch Health Companies Inc.
- Grupo Ferrer Internacional, S.A.
- SERB
Recent Developments
-
In December, 2024, Neurelis, Inc. presented findings at the American Epilepsy Society Annual Meeting, showcasing ten posters on immediate-use seizure medication for seizure clusters, including analyses of VALTOCO and its broader implications.
Acute Repetitive Seizures Market Report Scope
|
Report Attribute
|
Details
|
|
Market size value in 2025
|
USD 3.56 billion
|
|
Revenue forecast in 2030
|
USD 6.48 billion
|
|
Growth rate
|
CAGR of 12.7% from 2025 to 2030
|
|
Base year for estimation
|
2024
|
|
Historical data
|
2018 - 2023
|
|
Forecast period
|
2025 - 2030
|
|
Quantitative units
|
Revenue in USD million/billion and CAGR from 2025 to 2030
|
|
Report coverage
|
Revenue forecast, company ranking, competitive landscape, growth factors, trends
|
|
Segments covered
|
Product, region
|
|
Regional scope
|
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
|
|
Country scope
|
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, India, Australia, South Korea, Thailand, Brazil, Argentina, South Africa, Saudi Arabia, UAE, Kuwait
|
|
Key companies profiled
|
UCB S.A., Belgium; Neurelis, Inc.; Bausch Health Companies Inc.; Grupo Ferrer Internacional, S.A.; SERB
|
|
Customization scope
|
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
|
|
Pricing and purchase options
|
Avail customized purchase options to meet your exact research needs. Explore purchase options
|
Global Acute Repetitive Seizures Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global acute repetitive seizures market report based on product and region:
-
Product Outlook (Revenue in USD Million, 2018 - 2030)
-
USL-261
-
NRL-1
-
AZ-002
-
Diastat Rectal Gel
-
Others
-
Regional Outlook (Revenue in USD Million, 2018 - 2030)
-
North America
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
Latin America
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait















