Alpha Emitter Market Size & Trends
The global alpha emitter market size was valued at USD 678.41 million in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 11.44% from 2024 to 2030. The growing number of patients with cardiac & cancer ailments and increased awareness about the potential benefits of targeted alpha therapy are the key factors driving the market growth. Moreover, increasing product approvals by the regulatory authorities and growing investment in research and development of alpha emitter products are projected to propel the market during the study period.
The outbreak of the COVID-19 pandemic significantly impacted the alpha emitter market. As a result of reduced face-to-face interactions with physicians during the initial phase of the pandemic, fewer patients were referred to nuclear medicine for both diagnosis and treatment, with a somewhat milder impact observed in the case of oncological patients. According to a survey published in the PMC Journal in September 2020, a total of 434 responses from 72 countries affirmed a noteworthy decline in nuclear medicine procedures, with diagnostic procedures declining by over 50% and therapeutic procedures by around 40% during the COVID-19 outbreak. However, as the population adapts to the pandemic, the need for cancer care is anticipated surge, which in turn is projected to have significant impact on the market.
Moreover, increased awareness of the potential advantages associated with targeted alpha emitters, coupled with a substantial number of patients affected by various cancer types such as ovarian cancer, pancreatic cancer, lymphoma, and melanoma, are expected to provide a significant boost to market growth. For instance, according to the projections by the International Agency for Research on Cancer (IARC), by the year 2040, the global burden of cancer is expected to increase to 27.5 million new cancer cases and 16.3 million cancer-related deaths, primarily driven by population growth and aging. In addition approximately 70% of cancer-related deaths occur in low- and middle-income countries. As a result, the growing prevalence of cancer and cardiovascular diseases is expected to lead to an increased demand for alpha emitter for the treatment of these medical conditions.
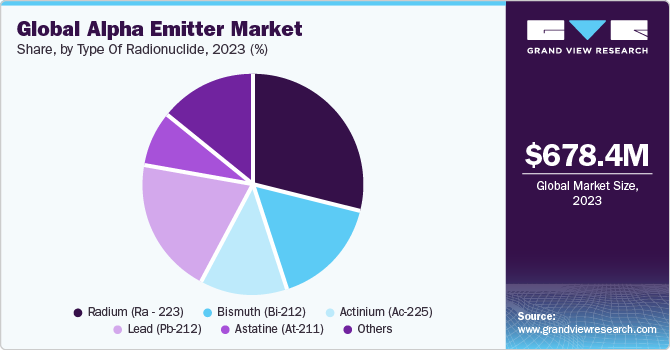
Type Of Radionuclide Insights
Based on the type of radionuclide, the market is segmented into Astatine, Radium, Actinium, Lead, Bismuth, other types of radionuclides. The radium segment held the largest market share in 2023, owing to the growing focus on research and development aimed at addressing metastatic castration-resistant prostate cancer. According to an article published in August 2022 on the Radiation journal, a researcher affiliated with Kindai University in Japan delved into the most effective approach for Radium-223 therapy in the context of Metastatic Castration-Resistant Prostate Cancer. The study findings pointed to a significant improvement in overall survival (OS) with early Ra-223 administration, suggesting potential benefits in administering Ra-223 before novel hormonal or anticancer agents.
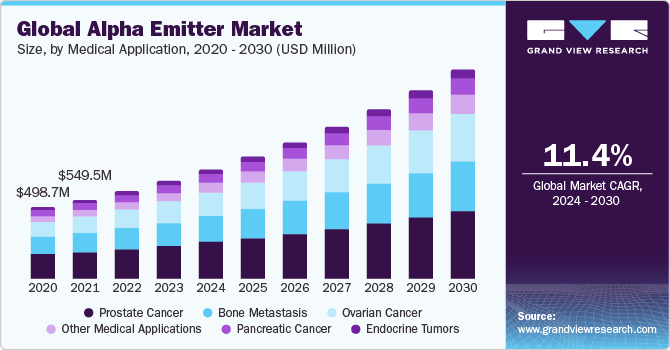
Medical Application Insights
On the basis of medical application, the market is segmented into prostate cancer, bone metastasis, ovarian cancer, pancreatic cancer, endocrine tumors, and other medical applications. The prostate cancer segment accounted for the largest revenue share in 2023, owing to the heightened focus on research and development in the realm of targeted alpha-particle-emitting radiopharmaceuticals as a therapeutic option for individuals with prostate cancer is becoming increasingly prominent. For instance, according to an article published on NCBI in April 2021, publication Information, researchers from Wake Forest University Health Sciences in North Carolina, United States, and the University of Iowa in the United States have revealed that radium-223 dichloride, an alpha-particle-emitting radiopharmaceutical, enhances the total survival rates of prostate cancer patients with bone metastases.
Regional Insights
North America was one of the major markets for alpha emitter in 2023. The rise in the use of non-organic growth strategies, such as partnerships among key market players, is seen as a contributing factor. This is expected to fuel market growth in the region throughout the forecast period, specifically in the development and production of innovative radiopharmaceuticals for cancer treatment. As an illustration, on January 1, 2023, NorthStar Medical Radioisotopes (NMR), a global company specializing in the development, manufacturing, and commercialization of radiopharmaceuticals for therapeutic purposes and medical imaging, joined forces with Inhibrx, Inc., a clinical-stage biotechnology firm, to collaborate on the creation and production of groundbreaking radiopharmaceuticals for cancer treatment.
Key Companies & Market Share Insights
Key players operating in the market are Actinium Pharmaceutical Inc., Alpha Tau Medical Ltd, Bayer AG, Fusion Pharmaceuticals, IBA Radiopharma Solutions, RadioMedix Inc., Telix Pharmaceuticals Ltd. The market participants are constantly working towards new product development, M&A activities, and other strategic alliances to gain new market avenues. The following are some instances of such initiatives:
-
In January 4, 2023, Orano Med, a pharmaceutical company, disclosed that it had administered the first dose of 212Pb-GRPR in a Phase 1 clinical trial. 212Pb-GRPR is an alpha radioligand therapy featuring lead-212, designed for patients with solid tumors that exhibit the gastrin-releasing peptide receptor.
-
In June 2022, Alpha Tau Medical Ltd., a medical technology firm, made an announcement regarding the approval of its Investigational Device Exemption (IDE) application by the U.S. Food and Drug Administration (FDA). This approval allows them to commence a pivotal, multi-center study for the treatment of recurrent cutaneous Squamous Cell Carcinoma (SCC) using their Alpha DaRT technology.














