- Home
- »
- Healthcare IT
- »
-
AI-based Clinical Trial Solutions For Patient Matching Market Report, 2030GVR Report cover
![AI-based Clinical Trial Solutions For Patient Matching Market Size, Share & Trends Report]()
AI-based Clinical Trial Solutions For Patient Matching Market Size, Share & Trends Analysis Report By Therapeutic Application, By End-use, By Region, And Segment Forecasts, 2022 - 2030
- Report ID: GVR-4-68039-982-8
- Number of Report Pages: 100
- Format: PDF, Horizon Databook
- Historical Range: 2016 - 2020
- Forecast Period: 2022 - 2030
- Industry: Healthcare
Report Overview
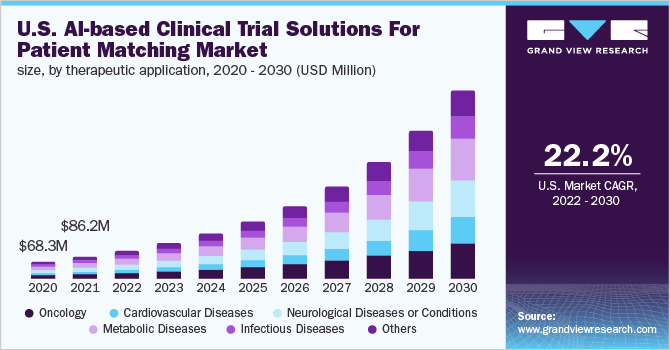
The global AI-based clinical trial solutions for patient matching market was valued at USD 230.6 million in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 26.7% from 2022 to 2030. The market growth is largely attributed to the inclusion of AI-powered solutions in conducting and managing clinical trials that can be beneficial for reducing clinical trial cycle time which further reduces the cost and also increases accuracy along with the productivity of the trial development. Increasing initiatives by the government, to promote the adoption of artificial intelligence (AI) technologies in the healthcare sector along with rising awareness about AI-based technology further fuels the market growth.
Adoption of the digitization in clinical and biomedical research is opening opportunities for AI-based clinical trials solution for the patient matching market. Key pharma companies are including advanced technological solutions for enhanced patient management and clinical trial results. AI-based clinical trial solutions increase patient recruitment by decreasing their population heterogeneity by harmonizing a large volumes of health information data from a wide array of sources and platforms such as medical imaging, electronic medical records (EMRs), and omics data.
These platforms allow selecting the groups of most appropriate patient population who have the highest chances of responding to the trial and higher likelihood of giving a quantifiable & measurable clinical endpoint. AI-based solution and systems can be utilized in examining patient health information records & clinical trial eligibility criteria and match them with recruiting clinical trial studies. AI-based solutions include natural language processing (NLP) algorithms which enhance the rate of matching between clinical trials and patient enrollment.
COVID-19 has transformed the perception of clinical trials. It has increased the penetration rate and utilization of these AI-based platforms for decreasing cost& time. Therefore, these factors are rising the adoption rate of artificial intelligence in pharmaceutical companies. For instance, major pharmaceutical players like Sanofi, Novartis, Johnson & Johnson, Pfizer, and Bayer are focusing on strategic alliances for the inclusion of AI-based solutions in medicine discovery & development and clinical trials process.
Government organizations of developed nations like the U.S. and Europe are providing funds and are simultaneously laying out a stringent regulatory framework to drive the adoption of AI-based solutions designed for clinical trial studies. Moreover, governments of the developing nations are also spreading awareness about AI-based clinical trial solutions amongst stakeholders to focus on discovering new medicines and accelerating patient recruitment as this will improve patient engagement & monitoring.
The increasing number of startups providing solutions for the AI-based recruiting patients, intended for the life science organizations in favor of their clinical studies are positively impacting the growth of the market. A large number of medicine manufacturers have to invest a substantial amount in the development of medicines to expand their product pipelines as many big vendors go off patent, which is one of the key factors for the growth of the clinical trials market.
Furthermore, growing initiatives by the public and private organization to support the adoption of AI-powered technological solutions in clinical trial studies is propelling the market growth. Pharma and biotech organizations are rapidly implementing AI-based platforms and solutions for supporting their clinical research studies. Corporations are adopting these solutions for enhancing recruitment, identification, engagement, and real-time monitoring of patients.
In developed countries, expenditure of healthcare IT holds a large share of healthcare expenses. Nations are incorporating AI-based tools to reduce costs and improve efficiency. For instance, NIH, in September 2022, launched the Bridge2AI program that will include members from diverse communities that will focus on generating different tools, resources, and data for building an AI approach.
However, risks related to data privacy may hamper the market in coming years. Adoption of AI-based technologies requires protecting the privacy and confidentiality of the large volumes of datasets of trial-related information. Most healthcare organizations are moving to the cloud for storing and managing petabytes of sensitive data. The cloud model allows users to access data from anywhere and at any time and thus, enabling a larger number of users to access the website, which increases the chances of cybersecurity attacks. This factor might hamper the market growth in the coming future.
Therapeutic Application Insights
In 2021, based on therapeutic applications, oncology accounted for the highest revenue share of 23.2%. The growing incidence rate of cancer throughout the world is leading to an increase in the number of clinical trials and is thus, impacting the market positively. Moreover, leading pharma companies are teaming up with AI developing companies to adopt AI-based oncology tools designed for the development process of medicines. For instance, in January 2022, Deep Lens and Hematology-Oncology Associates of Central New York came into a partnership that will focus on the clinical trial program expansion of the medical trial program. The VIPER by Deep Lens will be utilized to classify the eligible patients for clinical trials by pre-screening all patients.
The development of new technologies further fuels segment growth. For instance, in June 2022, Survivor Net launched an AI-based clinical trial finder that connects patients who are in need with groundbreaking cancer research.
Besides, the cardiovascular disease (CVD) segment is expected to reveal the highest CAGR during the forecast period owing to the rising prevalence of CVD throughout the world. As per the data of NIH’s, “New global data analysis highlights”, February 2021, the increasing percentage of the global population and geriatric population has led to substantial growth of CVD cases. This has resulted in major companies focusing on the development of new clinical solutions for cardiovascular diseases.
AI is highly being adopted for R&D purposes and improving clinical trial solutions for the analysis of CVD with a better approach. Thus, driving the segment’s growth. In June 2022, Bristol Myers Squibb signed a deal of USD 80.0 million with AI developer Owkin to design and optimize their CVD medicine trials. The partnership will emphasize adopting ML technologies to optimize their clinical trial process.
End-Use Insights
In 2021, based on end-use, pharmaceutical companies accounted for the highest revenue share of 66.0% owing to the growing focus on better development of the bio-markers and diagnostics using AI-based technologies to identify the new medicine target and simplify the process of development. For instance, Linguamatics offered by IQVIA is NLP-based software that provides text mining solutions for pharma companies.
The growing pharmaceutical industry due to the increasing penetration of chronic diseases and the growing demand for personalized medicine is further fueling the market growth. In addition, a collaboration between pharmaceutical giants and AI vendors for implementing AI technology intended for the overall medicine discovery process is further impacting the market positively.
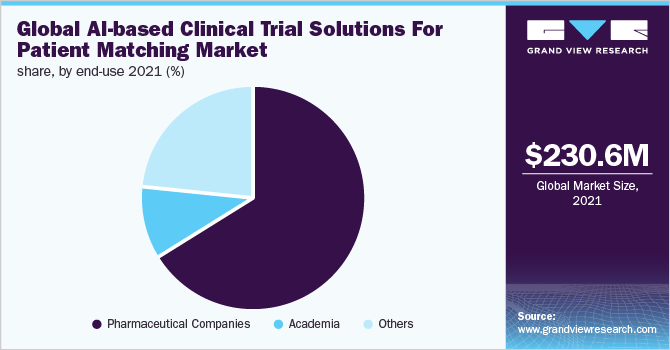
However, the others segment is projected to have the fastest CAGR in the forecast period. The growth can be attributed to the rising adoption of AI-based solutions by government agencies and CROs as these solutions reduce the cost of clinical trials by enhancing the quality of data, increasing compliance & retention of patients, and identifying the effectiveness of the treatment more efficiently. Furthermore, growing investment by the government and non-government agencies for the development of drugs and technology-driven clinical trials is further driving market growth.
Regional Insights
North America dominated the global AI-based clinical trial solutions for patient matching market and accounted for a revenue share of 43.8% in 2021. The growth of the market can be largely attributed to the presence of key players within the region and a growing number of AI-based startups. The region also has a large number of registered clinical trials which impacts the market positively.
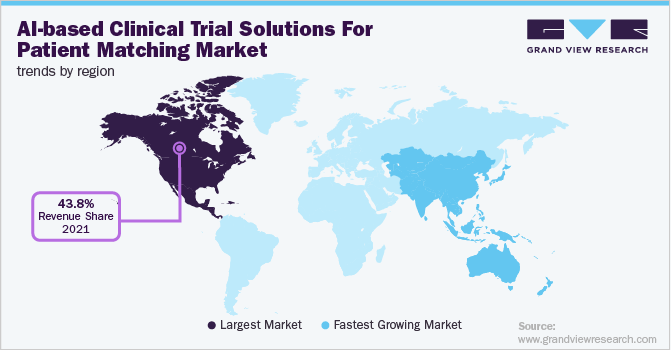
As per the data of the World Health Organization (WHO), from 1999-2021 U.S. conducted the highest number of clinical trials, i.e. about 157,618 trials. The growing interest in AI-based technologies and rising government initiatives for the adoption of AI-based technologies further fuel the region’s growth.
However, in the Asia Pacific, the market is expected to show the fastest CAGR during the forecast period owing to increasing penetration of AI-based clinical technologies and supportive government initiatives for adopting AI. A growing number of clinical trials within the region also impacts the market positively. As per the 2019’s data of NIH, Asia Pacific is becoming the hub for conducting cost-effective clinical trials. During the past 10 years, there has been a 7-fold increase in the registration of clinical trials. This is mainly due to the availability of highly skilled talents at lower cost, the presence of a large patient population, and the availability of competitive recruitment rates.
Key Companies & Market Share Insights
Increasing awareness about AI-based solutions and the rising adoption of such technologies are the key factors that are driving market growth. The rising demand has led to an increase in the number of start-ups that are expected to open new opportunities for the market. For instance, in February 2022, Qureight raised USD 1.50 million in seed funding for their AI-based platform which reduces the clinical trials’ process timeline and thus, decreases the cost of medicine.
Furthermore, strategic alliances in the form of mergers and acquisitions, collaborations, partnerships, etc. with other market players as well as increasing R&D in the area of AI-based technologies for clinical trials are some of the initiatives being undertaken by key market players, in order to increase their market shares. For instance, in April 2021, Deep Lens AI and Oregon Oncology Specialists entered a strategic collaboration to deploy a Deep Lens AI-powered clinical trial solution in identifying eligible patients for clinical trial studies. Some of the prominent players operating in the global AI-based clinical trial solutions for the patient matching market include:
-
Unlearn.AI, Inc.
-
Antidote Technologies, Inc.
-
Deep6.ai
-
Mendel.ai
-
Aris Global
-
Deep Lens AI
-
AmerisourceBergen Corporation
-
Koneksa
-
Microsoft Corporation
-
GNS Healthcare
AI-based Clinical Trial Solutions For Patient Matching Market Report Scope
Report Attribute
Details
The market size value in 2022
USD 290.7 million
The revenue forecast in 2030
USD 1.9 billion
Growth Rate
CAGR of 26.7% from 2022 to 2030
The base year for estimation
2021
Actual estimates/Historical data
2016 - 2020
Forecast period
2022 - 2030
Quantitative units
Revenue in USD million/billion, CAGR from 2022 to 2030
Report coverage
Revenue forecast, company share, competitive landscape, growth factors, trends
Segment Covered
Therapeutic application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; U.K.; Germany; Spain; France; Italy; Russia; China; Japan; India; South Korea; Australia; Mexico; Brazil; Argentina; South Africa; Saudi Arabia; UAE
Key companies profiled
Unlearn.AI, Inc.; Antidote Technologies; Inc.; Deep6.ai; Mendel.ai; Aris Global; Deep Lens; AmerisourceBergen Corporation; Koneksa; Microsoft Corporation; GNS Healthcare
15% free customization scope (equivalent to 5-analyst working days)
If you need specific market information, which is not currently within the scope of the report, we will provide it to you as a part of customization
Customization scope
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global AI-based Clinical Trial Solutions For Patient Matching Market Segmentation
This report forecasts revenue growth at global, regional, & country levels and provides an analysis of industry trends in each of the sub-segments from 2016 to 2030. For this study, Grand View Research, Inc. has segmented the global AI-based clinical trial solutions for patient matching market report based on the therapeutic application, end use, and region:
-
Therapeutic Application Outlook (Revenue, USD Million, 2016 - 2030)
-
Oncology
-
Cardiovascular Diseases
-
Neurological Diseases or Conditions
-
Metabolic Diseases
-
Infectious Diseases
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2016 - 2030)
-
Pharmaceutical Companies
-
Academia
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2016 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
France
-
Italy
-
Spain
-
Russia
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
MEA
-
South Africa
-
UAE
-
Saudi Arabia
-
-
Frequently Asked Questions About This Report
b. The global AI-based clinical trial solutions for patient matching market size was estimated at USD 230.6 million in 2021 and is expected to reach USD 290.7 million in 2022.
b. The global AI-based clinical trial solutions for patient matching market is expected to grow at a compound annual growth rate of 26.7% from 2022 to 2030 to reach USD 1.9 billion by 2030.
b. North America dominated the AI-based clinical trial solutions for patient matching market with a share of 43.8% in 2021. The growth of the market can be largely attributed to presence of key players within the region and growing number of AI-based startups. The region also has large number of registered clinical trials which impacts the market positively.
b. Some key players operating in the AI-based clinical trial solutions for patient matching market include Unlearn.AI, Inc., Antidote Technologies, Inc., Deep6.ai, Mendel.ai, Aris Global, Deep Lens, AmeriSourceBergen Corporation, Koneksa, Microsoft Corporation, GNS Healthcare
b. The market growth is largely attributed to inclusion of AI-powered solutions in conducting and managing clinical trials that can be beneficial for reducing clinical trial cycle time which further reduces the cost and also increases accuracy and productivity of the trial development.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





