- Home
- »
- Biotechnology
- »
-
Bone Morphogenetic Protein Market Size Report, 2030GVR Report cover
![Bone Morphogenetic Protein Market Size, Share & Trends Report]()
Bone Morphogenetic Protein Market (2025 - 2030) Size, Share & Trends Analysis Report By Type (rhBMP-2, rhBMP-7), By Application (Spinal Fusion, Trauma, Reconstruction, Oral Maxillofacial), By Region, And Segment Forecasts
- Report ID: GVR-1-68038-226-6
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Bone Morphogenetic Protein Market Summary
The global bone morphogenetic protein market size was estimated at USD 341.2 million in 2024 and is projected to reach USD 459.9 million by 2030, growing at a CAGR of 4.93% from 2025 to 2030. The growing demand for minimally invasive procedures, rising incidence of injuries, such as sports injuries & accident injuries, and increasing target population are some of the factors anticipated to fuel the market growth over the forecast period.
Key Market Trends & Insights
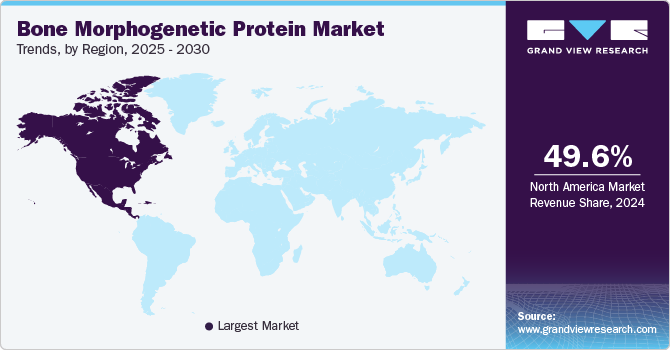
- The North America bone morphogenetic protein market held the largest market share of 49.6% in the global market in 2024.
- The Asia Pacific bone morphogenetic protein market held highest CAGR of 6.03% over the forecast period.
- Based on type, the rhBMP-2 segment held the largest market share of 73.9% in 2024 and is anticipated to grow at the higher CAGR from 2025 to 2030.
- Based on application, the spinal fusion segment held the largest market share of 50.3% in 2024.
Market Size & Forecast
- 2024 Market Size: USD 341.2 Million
- 2030 Projected Market Size: USD 459.9 Million
- CAGR (2025-2030): 4.93%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
BMPs, particularly recombinant BMPs like rhBMP-2, are valuable in orthopedic and reconstructive surgeries because they can promote bone healing and regeneration without requiring large grafts or invasive techniques. They help stimulate the body's natural healing processes, encouraging bone formation and fusion, which is essential in spinal fusions, trauma repairs, and reconstructive surgeries. As more surgical techniques focus on limiting the surgical footprint and recovery time, BMPs offer a solution that enhances outcomes in a minimally invasive context.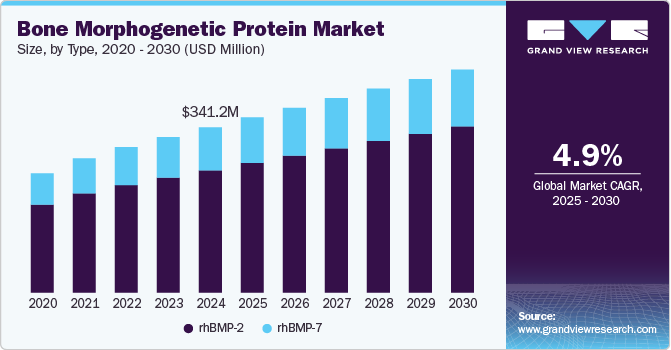
Moreover, several key benefits of BMPs in sports-related injuries drive the demand for them within this industry. Sports injuries often involve bone fractures, ligament tears, or joint damage, which can require surgical intervention to restore function and stability. According to the National Safety Council, in 2023, around 3.7 million people sought treatment for sports-related injuries in emergency rooms, with activities such as exercise, cycling, and basketball leading to the most injuries. BMPs have emerged as a promising treatment option for sports-related injuries as they promote bone healing and regeneration. The use of BMPs in such treatments can accelerate bone formation, improve healing outcomes, and reduce recovery times.
Furthermore, higher incidence of bone-related disorders is one of the leading drivers of the market. BMPs are often used in procedures such as spinal fusions and fracture healing to promote bone growth and recovery, making them highly relevant for the elderly. Moreover, spinal degeneration is a common problem in older adults due to the natural wear and tear of the spine. Degenerative disc disease, arthritis, and spinal stenosis often necessitate spinal fusion surgeries, where BMPs are frequently used to enhance the healing of vertebrae by stimulating new bone formation.
Market Concentration & Characteristics
The bone morphogenetic protein industry has seen significant growth in recent years. The development of advanced carriers for Bone Morphogenic Proteins (BMPs), such as biodegradable scaffolds, hydrogels, and nanoparticles, is a major technology focus. These delivery systems aim to enhance the localized and sustained release of BMPs, improving bone regeneration while minimizing off-target effects.
In the Bone Morphogenetic Protein industry, partnerships and collaboration activities are moderately established. For instance, in October 2023, Qkine appointed Clinisciences its exclusive European distribution partner to expand access to its high-purity, animal-free bioactive proteins. This partnership aims to support the growing European market for regenerative medicine and stem cell research by providing reliable supply and technical support for Qkine's products.
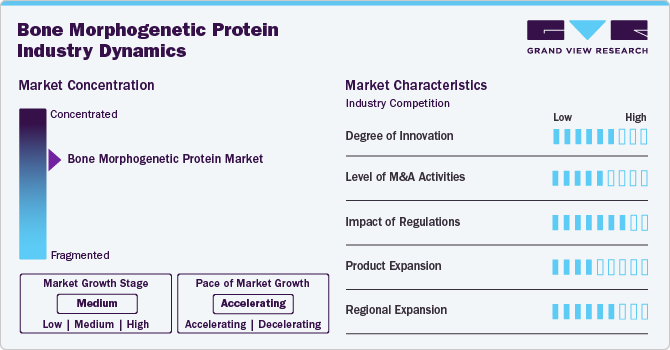
Regulations significantly impact the bone morphogenetic protein market. BMPs are regulated as biologics by agencies like the FDA and the European Medicines Agency (EMA). Products like rhBMP-2 have faced rigorous evaluation due to potential complications and long-term safety concerns. Regulatory bodies require robust clinical data to assess the risk-benefit profile, especially in cases involving high doses or off-label use of BMPs.
The bone morphogenetic protein industry has seen moderate growth in product expansion. This includes the development of specialized products for specific applications, for instance, in August 2022, Orthofix Medical and CGBio announced a strategic partnership to develop and commercialize Novosis, a recombinant human bone morphogenetic protein-2 (rhBMP-2) bone graft solution, for the U.S. and Canadian markets.
The bone morphogenetic protein industry is experiencing a moderate level of regional expansion, indicating rapid growth and increasing market presence across different geographic regions. regional market expansion for BMP is driven by factors like healthcare infrastructure development, increasing orthopedic procedures, and an aging population globally.
Type Insights
The rhBMP-2 segment held the largest market share of 73.9% in 2024 and is anticipated to grow at the higher CAGR from 2025 to 2030. The increasing focus on improving the delivery and retention of rhBMP-2 at surgical sites, the development of advanced solutions, and efforts by market players to increase their presence in various markets, which is increasing the availability of this protein globally, are driving its demand.
The advancements in biomaterials have aimed to improve the delivery and retention of rhBMP-2 at surgical sites. For instance, in September 2023, researchers developed a biocompatible, 3D Polycaprolactone (PCL) melt electro-written membrane that mimics the periosteum, which is crucial for bone regeneration. This membrane is functionalized with a poly (ethyl acrylate) coating to release low doses of recombinant human BMP-2 (rhBMP-2), demonstrating sustained osteoinduction in vitro and effective healing in vivo.
rhBMP-7 segment is expected to grow at a moderate CAGR from 2025 to 2030. As healthcare professionals increasingly look for alternatives to autografts for bone repair, rhBMP-7's ability to promote healing without the complications associated with harvesting grafts could drive its adoption in clinical practice. Similarly, the combination of orthopedic and neurological uses for rhBMP-7 is creating the way for new treatments that can help with complicated health issues affecting both bones and nerves.
Application Insights
The spinal fusion segment held the largest market share of 50.3% in 2024. The growth is attributed to the development of effective and efficient solutions, the increasing acceptance of these proteins over conventional treatments, and the ongoing research initiatives that strengthen their position in the market. The use of recombinant human bone morphogenetic proteins has emerged as a significant advancement in enhancing the success of spinal fusion surgeries. These proteins play a crucial role in osteoinduction, stimulating the differentiation of mesenchymal stem cells into osteoblasts, which are essential for new bone formation. Incorporating rhBMPs into surgical protocols has improved fusion rates significantly compared to traditional methods that relied solely on autografts or allografts.
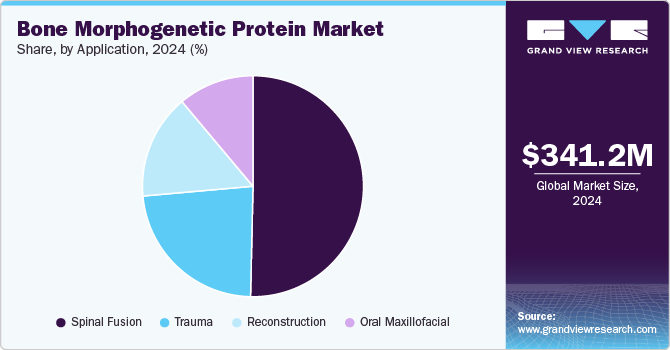
Trauma segment is expected to grow at the highest CAGR from 2025 to 2030. As the global population ages, the prevalence of bone-related injuries such as fractures, particularly hip fractures, is increasing. This has led to a rise in the number of orthopedic surgeries where BMPs are utilized to improve outcomes in bone regeneration.
Regional Insights
North America bone morphogenetic protein market held the largest market share of 49.6% in the global market in 2024. This can be attributed to the growing prevalence of bone disorders such as fractures, spinal fusions, & osteoporosis, and the surge in the demand for advanced treatments, and technological advancements in BMP formulations.
U.S. Bone Morphogenetic Protein Market Trends
The bone morphogenetic protein market in the U.S. is expected to grow over the forecast period. Regional growth can be attributed to the increasing incidence of bone disorders such as osteoarthritis and increasing demand for bone graft substitutes in spinal fusion and trauma surgeries is expected to drive the market.
Europe Bone Morphogenetic Protein Market Trends
Europe bone morphogenetic protein market was identified as a lucrative region in this industry. The aging population in Europe contributes to a higher prevalence of conditions such as osteoarthritis, fractures, and osteoporosis, where BMPs play a significant role in promoting bone healing and regeneration.
The bone morphogenetic protein market in the UK held a significant share in 2024. Technological advancements, such as integrating BMPs with 3D-printed scaffolds and bioengineered tissue products, further enhance treatment outcomes, making the market more competitive in the UK.
France bone morphogenetic protein market is expected to grow significantly over the forecast period. Academic institutions, research centers, and private companies are actively developing new applications of BMPs, enhancing clinical outcomes and boosting market growth.
The bone morphogenetic protein market in Germany is anticipated to grow significantly over the forecast period. The well-established healthcare infrastructure, robust R&D, and access to advanced medical technologies create an ideal environment for innovation and the adoption of BMP in treating bone-related disorders.
Asia Pacific Bone Morphogenetic Protein Market Trends
The Asia Pacific bone morphogenetic protein market held highest CAGR of 6.03% over the forecast period. Key factors driving industry growth include growing access to advanced technologies, a large patient pool, and improvement in healthcare infrastructure. The increasing adoption of BMP in developing markets by healthcare professionals is also a significant factor driving the region’s growth.
The bone morphogenetic protein market in China is expected to grow over the forecast period. Expansion of the biopharmaceuticals market is driving the bone morphogenetic protein market in China.
Japan bone morphogenetic protein market is witnessing rapid growth over the forecast period. The presence of numerous pharmaceutical & biotechnology companies, increasing health consciousness, and a demographic challenge with the oldest population in the world are expected to boost the demand for bone morphogenetic protein for various research activities and clinical purposes.
MEA Bone Morphogenetic Protein Market Trends
The Middle East and Africa bone morphogenetic protein marketis expected to witness steady growth over the forecast period owing to heterogeneity in the economic progress of countries.
Saudi Arabia bone morphogenetic protein market is expected to grow over the forecast period. This growth is likely to be driven by continuous investments in healthcare infrastructure and technological advancements. Favorable pricing strategies, along with the growing focus on medical research and innovation in regenerative medicine.
The bone morphogenetic protein market in Kuwait is anticipated to grow moderately over the forecast period. This is attributed to its evolving healthcare infrastructure and increasing awareness regarding orthopedic & spinal disorders.
Key Bone Morphogenetic Protein Company Insights
The market players operating in the bone morphogenetic protein market are adopting product approval to increase the reach of their products in the market and improve the availability of their products in diverse geographical areas, along with expansion as a strategy to enhance production activities. In addition, several market players are acquiring smaller players to strengthen their market position. This strategy enables companies to increase their capabilities, expand their product portfolios, and improve their competencies.
Key Bone Morphogenetic Protein Companies:
The following are the leading companies in the bone morphogenetic protein market. These companies collectively hold the largest market share and dictate industry trends.
- Medtronic plc
- Cellumed Co., Ltd.
- Cell Guidance Systems LLC
- Merck KGaA
- Prospec-Tany Technogene Ltd.
- Qkine Ltd.
- Bio-Techne.
- Thermo Fisher Scientific Inc.
- Proteintech Group, Inc.
- STEMCELL Technologies
Recent Development
-
In March 2024, CGBio gained FDA approval for its Novosis putty solution. Novosis is designed to provide a moldable, bioactive bone graft material that enhances surgical outcomes. It integrates a bone-forming protein known as recombinant human bone morphogenetic protein 2 (rhBMP-2; Nebotermin) with ceramic scaffolds to enhance the process of bone growth.
-
In March 2024, Merck KGaA transitioned its eCommerce operations in Indonesia from SigmaAldrich to the global SigmaAldrich.com platform. This would enable it to offer customers enhanced features, such as product pricing in Indonesian Rupiah (IDR); improved search capabilities; a comprehensive product portfolio, and real-time stock information, facilitating a streamlined shopping experience.
-
In August 2023, Qkine announced a distribution partnership with Biogenuix in India to enhance the availability of its animal-free, bioactive proteins for stem cell and organoid culture. This partnership aims to support the growing Indian market for regenerative medicine, providing access to Qkine's full product portfolio while ensuring high-quality customer service.
-
In April 2023, Qkine Ltd. launched a new biomanufacturing and R&D facility in Cambridge, UK, to enhance its production of high-purity, animal-free bioactive proteins. This expansion aims to meet the increasing global demand for proteins used in stem cell research, cultivated meat, and regenerative medicine, driving innovation and improving scientific reproducibility.
Bone Morphogenetic Protein Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 361.6 million
Revenue forecast in 2030
USD 459.9 million
Growth rate
CAGR of 4.93% from 2025 to 2030
Historical data
2018 - 2024
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, application, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Argentina; South Africa; Saudi Arabia, UAE; Kuwait
Key companies profiled
Medtronic plc; Cellumed Co., Ltd.; Cell Guidance Systems LLC; Merck KGaA; Prospec-Tany Technogene Ltd.; Qkine Ltd.; Bio-Techne.; Thermo Fisher Scientific Inc.; Proteintech Group, Inc.; STEMCELL Technologies; Sartorius Stedim Biotech
Customization scope
Free report customization (equivalent up to 8 analyst's working days) with purchase. Addition or alteration to country, regional & segment scope.
Global Bone Morphogenetic Protein Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the global bone morphogenetic protein market based on type, application, and region.
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
rhBMP-2
-
rhBMP-7
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Spinal Fusion
-
Trauma
-
Reconstruction
-
Oral Maxillofacial
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global bone morphogenetic protein market size was estimated at USD 341.2 million in 2024 and is expected to reach USD 361.6 million in 2025.
b. The global bone morphogenetic protein market is expected to grow at a compound annual growth rate of 4.93% from 2025 to 2030 to reach USD 459.9 million by 2030.
b. rhBMP 2 dominated the bone morphogenetic protein market with a share of 73.93% in 2024. This is attributable to their extensive osteoinductive properties. Due to the longstanding approval and wide range of applications, this BMP type holds a dominant market share.
b. Some key players operating in the bone morphogenetic protein market include Thermo Fisher Scientific Inc.; STEMCELL Technologies; Merck KGaA; Greiner Bio-One International GmbH; Corning Incorporated; Wilson Wolf; DWK Life Sciences; Cell Culture Company, LLC; VWR International, LLC.; Danaher, Sartorius Stedim Biotech
b. Key factors that are driving the market growth include growing demand for minimally invasive medical procedures, increasing incidence of sports-related injuries, and increasing target population.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










