- Home
- »
- Medical Devices
- »
-
Canada Medical Writing Market Size & Share Report, 2030GVR Report cover
![Canada Medical Writing Market Size, Share & Trends Report]()
Canada Medical Writing Market (2023 - 2030) Size, Share & Trends Analysis Report By Type (Clinical, Regulatory, Scientific), By Application (Medical Journalism, Medico Marketing), By End-use, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-044-5
- Number of Report Pages: 67
- Format: PDF
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Report Overview
The Canada medical writing market was valued at USD 119.9 million in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 10.36% from 2023 to 2030. The growth is driven by factors such as increased R&D investments by the key players. Prominent healthcare & life science companies spend a large share of their revenues on R&D activities to maintain their position in the market by introducing innovative products. For instance, Roche, Novartis, and Merck invest around 20%, 18%, and 17% of their annual revenues in R&D, respectively.
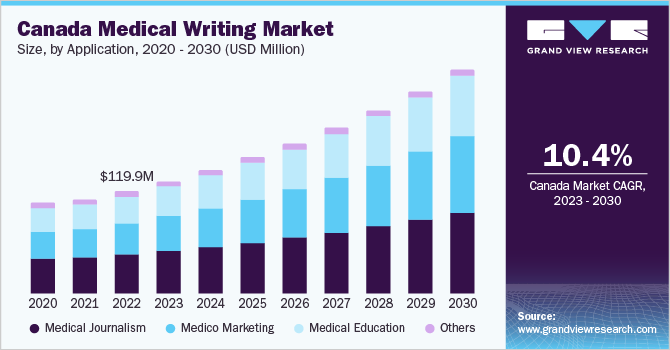
Furthermore, increasing government initiatives to support clinical trials are expected to propel the demand for clinical trial writing in the coming years. For instance, in June 2022, the Canadian government introduced the Clinical Trials Fund (CTF) backed by a USD 250 million investment over 3 years in Budget 2021 for the Canadian Institutes of Health Research (CIHR). As an important part of Canada's Biomanufacturing and Life Sciences Strategy, the CTF will support the training of new clinical researchers and support the clinical trials infrastructure in Canada.
Social media has become an important aspect of the communication and marketing strategies of many companies, including healthcare providers. Internet and social media are now no longer optional. Social networking and advertising are now being used to engage with patients as part of the digital marketing strategy. According to a 2020 Canadian Internet Use Survey, approximately 69% of internet users have searched online for health-related information. Thus, the rapid expansion of health- and wellness-related blogs is anticipated to increase the demand for scientific writers.
To increase patient outreach, pharmaceutical companies have recently modified their advertising strategies. Also, the pharmaceutical and biotechnology industries have been forced to reevaluate their marketing approaches due to the growing impact of social media on consumer decisions. So, it is anticipated that the demand for scientific writing will increase as a result of the strong growth of blogs connected to health and wellbeing.
The COVID-19 pandemic halted research and development and clinical trials. In addition, many pharmaceutical companies have reduced their investments due to limited financial resources. This hindered the growth of the market. However, the increasing number of clinical trials is expected to increase the demand for medical writers. This will present a substantial opportunity for clinical writing service providers during the forecast period.
Type Insights
Based on type, the market is segmented into clinical writing, regulatory writing, scientific writing, and others. The clinical writing segment dominated the market with a share of 37.6% in 2022. This can be attributed to factors such as Clinical Research Organizations (CROs) establishing medical writing as a feasible extension to their current service portfolio owing to their access to a sizable amount of clinical trial data.
Clinical writing is used by health professionals regularly, which includes health charts and forms. A clinical writer must have a thorough knowledge of medical language and culture. An understanding of the target reader & purpose of the writing is required for effective clinical writing. It provides a direction to patient care through accurate, crisp, and factual documentation.
On the other hand, the regulatory writing segment is projected to register the fastest CAGR over the forecast period. Regulatory writers work closely with experts in fields such as data management, biostatistics, regulatory affairs, and clinical trial management. They work with the internal team and sponsors of clinical trials for developing protocols for easier execution. Regulatory writers review Standard Operating Procedures (SAP) and prepare a Clinical Study Report (CSR) by regulatory requirements.
Application Insights
In terms of application, the Canada medical writing market is segmented into medical journalism, medico marketing, medical education, and others. The medical journalism segment accounted for the maximum revenue share in 2022. Medical journalism is mainly concerned with investigating, reporting, and communicating medical issues to a wide audience. Rapid advancements in the field of medicine and the growing importance of medical information have increased the need for writers who are capable of comprehending & communicating medical information through their writing.
In 2022, the Canadian Press (CP) and the Canadian Medical Association (CMA) launched an initiative to support health journalism across the country. The CMA and The CP have formed a partnership to strengthen health journalism across Canada. This initiative will enable The CP to provide a variety of stories covering the entire spectrum of health and health care in Canada, as well as how these issues affect Canadians' lives. All such initiatives are contributing to the growth of the segment in Canada.
On the other hand, the medico marketing segment is expected to witness lucrative growth during the forecast period. Medico marketing writing involves the writing of marketing content for drugs and other products, and the ever-changing product mix in the biotechnology and pharmaceutical industries acts as a driving force for improved marketing practices.
End-use Insights
The contract research organizations (CROs) & others segment dominated the market with a share of 68.1% in 2022. The segment will remain dominant throughout the forecast period. A rise in the number of CROs across Canada is driving the segment’s growth. Innovaderm, IQVIA Holdings Inc., dicentra, ICON, and Syreon are some of the major CROs operating in Canada. These top five organizations provide extensive clinical research services and digital solutions for a wide range of indications to enhance patient care at facilities in Canada and around the world.
Furthermore, the CROs are also expected to register the fastest CAGR over the forecast period owing to increased utilization of medical writing by the CROs. CROs play a diversified role in drug development in the pharmaceutical industry. An increase in biotechnology investments, mergers & collaborations, and new product development strategies are shaping the way research studies are being performed and creating opportunities for medical writing.
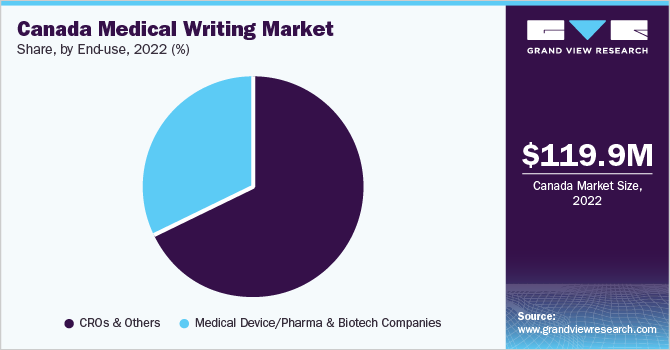
Medical device, pharmaceutical, and biotechnology companies frequently hire medical writers or medical journalists. The nature of writing can vary from company to company. Medical writers are mainly involved in the preparation of regulatory documents, scientific documentation, and documentation during clinical trials for drug development, among others. Established pharmaceutical companies have separate departments for medical writing purposes. In addition, various companies outsource medical writing services.
Key Companies & Market Share Insights
Industry rivalry is expected to be moderately high during the forecast period. Geographic expansion, partnerships, and an increase in service offerings are some of the strategies being implemented by market players. For instance, in September 2021, Certara announced the launch of Synchrogenix Writer, a regulatory SaaS product that accelerates the review and authoring of patient narratives. Some prominent players in the Canada medical writing market include:
-
Premier Research
-
Freyr
-
IQVIA Holdings Inc.
-
Certara Inc.
-
Quanticate
-
Soterius, Inc.
-
Parexel International Corporation
Canada Medical Writing Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 131.8 million
Revenue forecast in 2030
USD 262.7 million
Growth Rate
CAGR of 10.36% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, application, end-use
Country scope
Canada
Key companies profiled
Premier Research; Freyr; IQVIA Holdings Inc.; Certara Inc.; Quanticate; Soterius, Inc.; Parexel International Corporation
Customization scope
Free report customization (equivalent to up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
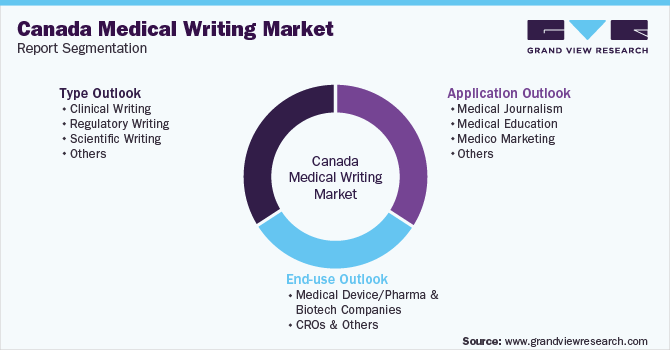
Canada Medical Writing Market Report Segmentation
This report forecasts revenue growth at the country level and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the Canada medical writing market report based on type, application, and end-use:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Clinical Writing
-
Regulatory Writing
-
Scientific Writing
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Medical Journalism
-
Medical Education
-
Medico Marketing
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Medical Device/Pharma & Biotech Companies
-
CROs & Others
-
Frequently Asked Questions About This Report
b. The Canada medical writing market size was estimated at USD 119.9 million in 2022 and is expected to reach USD 131.8 million in 2023.
b. The Canada medical writing market is expected to grow at a compound annual growth rate of 10.36% from 2023 to 2030 to reach USD 262.7 million by 2030.
b. Medical Journalism dominated the Canada medical writing market with a share of 38.0% in 2022. This is attributable to rapid advancements in the field of medicine and the growing importance of medical information.
b. Some key players operating in the Canada medical writing market include Premier Research; Freyr; IQVIA Holdings Inc.; Certara, Inc.; Quanticate; Soterius, Inc.; and Parexel International Corporation
b. Key factors that are driving the market growth include robust growth in CRO outsourcing, Increased R&D investments by market players, and growing penetration of internet and digital media in Canada.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










