- Home
- »
- Medical Devices
- »
-
Cardiovascular Repair And Reconstruction Devices Market Report, 2030GVR Report cover
![Cardiovascular Repair And Reconstruction Devices Market Size, Share & Trends Report]()
Cardiovascular Repair And Reconstruction Devices Market Size, Share & Trends Analysis Report By Product (Heart Valve Repair, Vascular Graft, Cardiovascular Patches), By Region (North America, Europe), And Segment Forecasts, 2024 - 2030
- Report ID: GVR-2-68038-156-6
- Number of Report Pages: 120
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Market Size & Trends
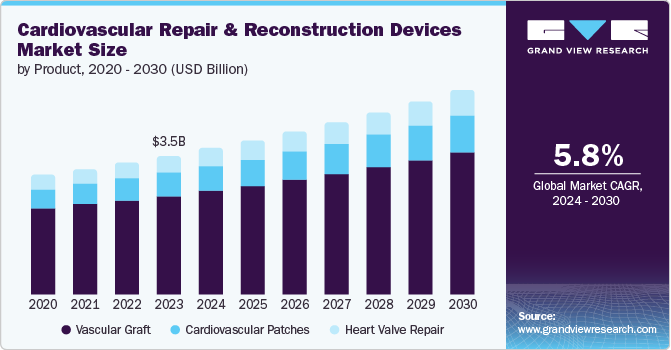
The global cardiovascular repair and reconstruction devices market size was valued at USD 3.52 billion in 2023 and is projected to grow at a CAGR of 5.8% from 2024 to 2030. The increasing prevalence of cardiovascular diseases worldwide, due to factors such as inactive lifestyles, poor diets, and aging populations. This has resulted in an increased need for cardiovascular repair and reconstruction devices. Innovations in device design and function, as well as improvements in surgical techniques, are enabling more effective and efficient treatments. Furthermore, expanding healthcare infrastructure in emerging economies facilitates greater access to such treatments.
The market is poised for substantial growth driven by emerging trends in healthcare, including artificial intelligence (AI) applications in diagnostics and treatment planning, advancements in regenerative medicine and tissue engineering, and the rise of personalized medicine and therapy. Moreover, the increasing significance of biochemicals as integral components of treatment regimens is expected to further fuel market expansion.
Increased investment in Research and Development (R&D) for peripheral arterial disease (PAD) holds significant promise for improving treatment efficacy and developing novel solutions. At present, angioplasty serves as the primary intervention for PAD. However, atherectomy offers another approach for addressing atherosclerotic plaque buildup. This focus on minimally invasive endovascular techniques to address arterial blockages is driving innovation in the medical device industry. This factor is anticipated to propel the market in the upcoming years.
Product insights
Vascular graft products dominated the market with a revenue share of 71.3% in 2023. This dominance is linked to the rising demand for less invasive procedures, which is driven by developments in clinical pharmacology, cell biology, vascular surgery, and molecular chemistry. The appeal of vascular graft procedures has been further enhanced by technological advancements such as ePTFE patches and transcatheter valves, making them particularly beneficial for treating Congenital Heart Defects (CHDs) such as ventricular septal defects, atrial septal defects, and double outlet ventricles. Furthermore, vascular grafts are widely preferred for use in pediatric procedures. These factors have collectively contributed to the expansion of this market segment.
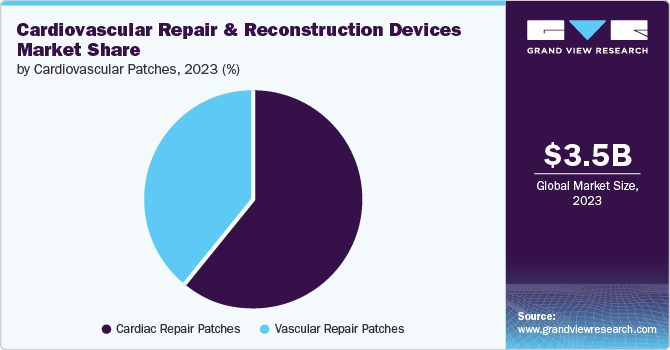
The valve repair segment is expected to grow fastest at a CAGR of 6.9% from 2024 to 2030. This growth is driven by advancements in the treatment of cardiovascular conditions related to heart valves. Valve repair devices have revolutionized treatment, leading to a reduction in postoperative complications, the introduction of minimally invasive procedures, improved patient outcomes through the use of novel materials, advancements in transcatheter techniques, and overall enhancement of cardiovascular interventions.
Regional Insights
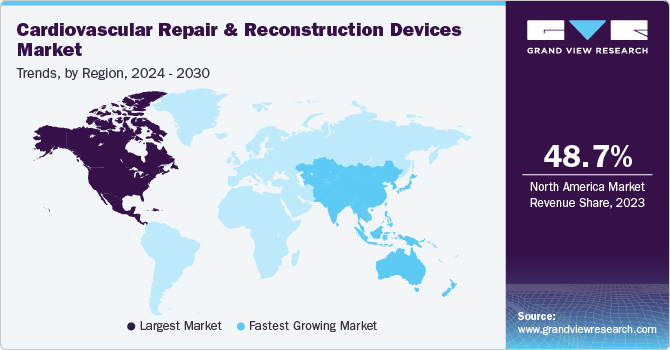
North America dominated the market with revenue share of 48.66% in 2023. Growing urbanization and technological advancement, rapidly growing markets, and rising disposable income are the factors attributed to the market's growth in the region.
U.S. Cardiovascular Repair And Reconstruction Devices Market Trends
The U.S. cardiovascular repair and reconstruction devices market held the major share of 87.8% in North America in 2023. This was driven by the growing incidence of heart disorders due to unhealthy lifestyle choices, leading to increased demand for cardiovascular disease monitoring and diagnostic devices.
Europe Cardiovascular Repair And Reconstruction Devices Market Trends
Europe cardiovascular repair and reconstruction devices market was identified as a lucrative region in 2023. This can be attributed to shifting consumer preferences and a well-established infrastructure, both of which have contributed to the market's growth in the region.
UK cardiovascular repair and reconstruction devices market is projected to experience significant growth in the forthcoming years as a result of enhanced regulatory standards, improved clinical evidence, and increased emphasis on safety and transparency.
Asia Pacific Cardiovascular Repair And Reconstruction Devices Market Trends
The Asia Pacific market for cardiovascular repair and reconstruction devices is expected to experience substantial growth in the coming years. This growth can be attributed to a large population, increasing demand, a growing number of elderly individuals, and higher disposable incomes.
The cardiovascular repair and reconstruction devices market in China held a substantial market share in 2023 owing to growing awareness about heart diseases. Increased medical tourism is also a factor in the country's market growth.
Key Cardiovascular Repair And Reconstruction Devices Company Insights
Some of the key companies in the cardiovascular repair and reconstruction devices market include Medtronic, BD, Terumo, and others. Organizations are focusing on increasing customer base to gain a competitive edge in the industry. Therefore, key players are taking several strategic initiatives, such as mergers and acquisitions, and partnerships with other major companies.
Key Cardiovascular Repair And Reconstruction Devices Companies:
The following are the leading companies in the cardiovascular repair and reconstruction devices market. These companies collectively hold the largest market share and dictate industry trends.
- Medtronic plc
- BD
- TERUMO CORPORATION
- W. L. Gore & Associates, Inc.
- Getinge
- Artivion, Inc
- Edwards Lifesciences Corporation
Recent Developments
-
In May 2024, MEACOR secured USD 15 million in Series A funding. This significant investment is expected to be utilized to advance the development of their groundbreaking transcatheter heart valve repair technology.
-
In January 2023, Terumo and Siemens Healthineers India partnered to strengthen cardiac care in India through collaborative interventions in the areas of physician training and development, access to advanced medical technologies, and improved penetration of Tier 2 and 3 cities.
Cardiovascular Repair And Reconstruction Devices Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 3.71 billion
Revenue forecast in 2030
USD 5.20 billion
Growth Rate
CAGR of 5.8% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; South Korea; Australia; Thailand; Brazil; Argentina; Saudi Arabia; UAE; Kuwait; South Africa
Key companies profiled
Medtronic plc; BD.; TERUMO CORPORATION; W. L. Gore & Associates, Inc.; Getinge; Artivion, Inc; Edwards Lifesciences Corporation
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Cardiovascular Repair And Reconstruction Devices Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global construction repair and reconstruction devices market report based on product and region:
-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Heart Valve Repair
-
Vascular Graft
-
Endovascular Stent Graft
-
Hemodialysis Access Graft
-
Peripheral Vascular Graft
-
-
Cardiovascular Patches
-
Cardiac Repair Patches
-
Atrial Septal Defect
-
Common Atrium
-
Defects of the Endocardial Cushion
-
Ventricular Septal Defect
-
Tetralogy of Fallot
-
Suture bleeding
-
-
Vascular Repair Patches
-
Carotid Endarterectomy
-
Anomalous Connection of The Pulmonary Veins
-
Transposition of the Great Vessels
-
Reconstruction of Portal and Superior Mesenteric Veins
-
Other Vascular Repair Applications
-
-
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
KSA
-
UAE
-
South Africa
-
Kuwait
-
-
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





