- Home
- »
- Biotechnology
- »
-
Carrier Screening Market Size, Share, Industry Report, 2033GVR Report cover
![Carrier Screening Market Size, Share & Trends Report]()
Carrier Screening Market (2026 - 2033) Size, Share & Trends Analysis Report By Medical Conditions (Spinal Muscular Atrophy, Cystic Fibrosis, Tay-Sachs, Gaucher Disease, Sickle Cell Disease), By Type, By Technology, By End Use, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-016-9
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2021 - 2025
- Forecast Period: 2026 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Carrier Screening Market Summary
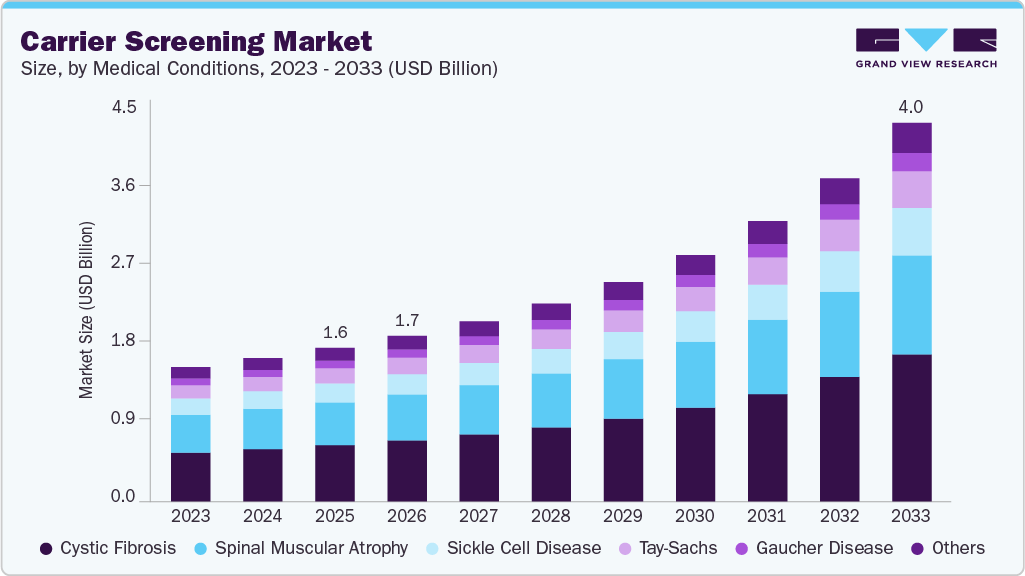
The global carrier screening market size was estimated at USD 1.64 billion in 2025 projected to reach USD 4.05 billion in 2033, growing at a CAGR of 12.55% from 2026 to 2033. The rising prevalence of genetic disorders, technological advancements in genetic testing, growing awareness and demand for personalized medicine are major factors driving the market’s growth.
Key Market Trends & Insights
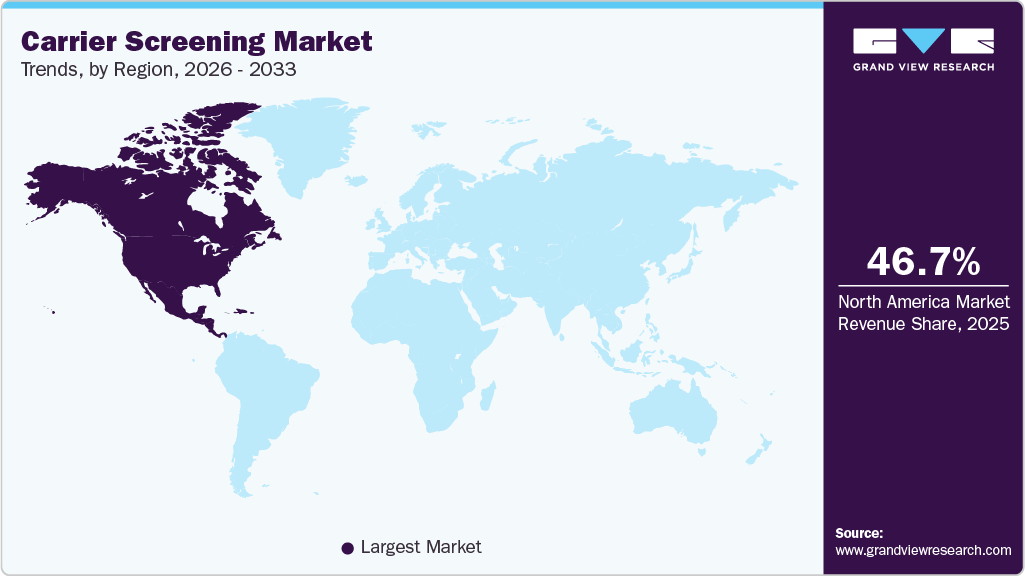
- North America carrier screening market dominated the global market and accounted for the largest revenue share of 46.73% in 2025.
- The U.S. led the North American market and held the largest revenue share in 2025.
- Based on medical conditions, the cystic fibrosis segment dominated the global market and accounted for the largest revenue share of 36.7% in 2025.
- Based on type, the expanded carrier screening segment held the largest revenue share of 74.9% in 2025.
- On the basis of technology, the DNA sequencing segment held the largest revenue share of 45.0% in 2025.
Market Size & Forecast
- 2025 Market Size: USD 1.64 Billion
- 2033 Projected Market Size: USD 4.05 Billion
- CAGR (2026-2033): 12.55%
- North America: Largest Market in 2025
For instance, according to the World Health Organization, around 10 in every 1,000 individuals are affected, translating to between 70 million and 80 million people worldwide living with such conditions. Sickle cell disease (SCD) alone impacts more than 100,000 people in the U.S., with a particularly high prevalence among people of African American origin.The growing technological advancement in screening techniques, particularly in next-generation sequencing (NGS) and bioinformatics, has transformed the carrier screening market by improving accuracy, speed, and affordability. Traditional genetic screening tests typically focused on a limited set of conditions, targeting specific populations or high-risk groups. In contrast, modern NGS-based panels screen hundreds of genetic mutations in a single run, providing a much broader and more comprehensive assessment. For instance, in February 2025, F. Hoffmann-La Roche AG (Switzerland) introduced a new category of next-generation sequencing by expansion (SBX) technology, which is playing a vital role in decoding complex diseases like cancer, immune disorders and neurodegenerative conditions. This technology uses high throughput sensor module along with synthetic molecules in order to determine the DNA sequence of a target molecule.
Moreover, the expansion of the carrier screening market is propelled by rising awareness of genetic risks and the global shift toward personalized medicine. Individuals and couples are becoming increasingly proactive in managing reproductive health, seeking genetic counseling along with screening. This reflects a growing understanding that carrier status has direct implications for family planning, pregnancy outcomes, and the health of future generations.
The foundation of carrier screening lies in raising awareness in order to improve health outcomes through early detection programs. Early programs such as Tay-Sachs disease screening in Ashkenazi Jewish communities demonstrated how education and testing could drastically reduce disease prevalence. Similarly, community-driven sickle cell screening efforts in African American populations underscored the importance of proactive engagement, though later government-led initiatives revealed the risks of poor implementation and misinformation. These historical lessons emphasize a broader trend toward personalized medicine, where individuals and communities increasingly seek genetic insights to make informed reproductive and healthcare decisions.
However, one of the major challenges in the market, as traditional short-read NGS struggles with complex regions of the genome, including repeat expansions, structural variants, methylation signatures, and high-homology genes. To address these gaps, innovations such as highly accurate long-read sequencing, including HiFi technologies, are emerging. For instance, in September 2025, PacBio launched its expanded PureTarget portfolio for high-throughput carrier screening, powered by HiFi sequencing technology. The solutions streamline workflows by consolidating multiple assays into a single scalable test, enabling accurate analysis of challenging genes.

Market Concentration & Characteristics
The carrier screening market demonstrates high innovation, driven by next‑generation sequencing (NGS) & whole‑exome sequencing, cost reduction & throughput increase, and expanded panels & broader disease coverage. The next‑generation sequencing (NGS) has enabled few known variants in few genes to comprehensive risk across all genes and populations.
Mergers and acquisitions increasingly shape this market, as large diagnostics players acquire startups with proprietary carrier screening technologies. The M&A activities are expanding technological capabilities, influencing pricing, and broadening access of testing. Additionally, increasing incidences of genetic diseases is influencing the market players to form mergers and acquisitions in order to enhance their service offerings and market reach.

Regulatory impact is high due to complex federal and state laws, clinical guidelines, reimbursement policies, and ethical considerations. For instance, in the United States, the Clinical Laboratory Improvement Amendments (CLIA) of 1988 established quality standards for all clinical laboratory testing, including genetic testing. The stringent requirements for test accuracy, reliability, and timeliness create a high barrier to entry for testing providers.
Product expansion across the globe is medium in the carrier screening market because of the technology focused such as seamless EMR integration, modular automation suites, long-read sequencing, non-invasive PGT. The seamless EMR integration develops integrated electronic ordering systems that allow physicians to easily order tests and receive results directly in a patient's electronic medical record (EMR).
North America plays a leading role in the adoption of carrier screening, with the U.S. driving much of the growth due to advanced healthcare infrastructure and the integration of genetic testing into prenatal care. Moreover, expanded carrier screening (ECS) is gradually gaining traction across Europe, particularly in private fertility clinics, while public healthcare systems remain cautious due to cost, equity, and resource considerations.
Medical Conditions Insights
On the basis of medical conditions, cystic fibrosis accounted for the largest market share of 36.69% in 2025 and expected fastest growth in forecast year. CF is one of the most common inherited disorders among populations of European descent, and professional medical societies, including the American College of Obstetricians and Gynecologists (ACOG), recommend universal screening for CF in all pregnancies. As a result, CF screening has become a cornerstone of targeted carrier screening panels and remains a key driver of market adoption. The market is further expanding as new technologies enable more accurate, accessible, and comprehensive detection of CF variants.
A wide range of carrier screening products include CF testing as a standard feature. For instance, Invitae Corporation’s Carrier Screen offers a tiered approach, with its most targeted panel covering cystic fibrosis (CF), spinal muscular atrophy (SMA), and Fragile X syndrome-the three universally recommended conditions. Similarly, broader expanded carrier screening panels, such as Fulgent Genetics’ Beacon787 or Myriad Genetics’ Foresight Carrier Screen, also incorporate CF among the hundreds of genes assessed, ensuring that couples receive this critical information regardless of whether they choose a targeted or expanded test. This consistency reflects CF’s established role as a benchmark condition in carrier screening.
Type Insights
Expanded carrier screening (ECS) is witnessing strong market growth, driven by new clinical guidelines, innovative product launches, and the rising adoption of large-scale screening programs worldwide. Traditionally, carrier screening was limited to specific conditions based on family history or ethnic background. However, ECS panels now allow for simultaneous testing of hundreds of genes associated with severe recessive and X-linked conditions, making reproductive risk assessment more comprehensive and inclusive.
Further, in March 2023, a significant milestone was reached when Myriad Genetics, Inc. announced its support for the first evidence-based ECS practice guidelines published by the National Society of Genetic Counselors (NSGC) in the Journal of Genetic Counseling. These guidelines represent an important shift toward standardizing how ECS is recommended and implemented, reinforcing its role in reproductive health decision-making.
Technology Insights
Based on technology, DNA sequencing accounted for the largest market share of 45.00% in 2025 and expected fastest growth in forecast year. DNA sequencing technologies, particularly next-generation sequencing (NGS), are reshaping the carrier screening market by enabling large-scale, accurate, and cost-efficient genetic analysis. Unlike traditional methods such as PCR and microarrays, which are limited to detecting a smaller number of variants, NGS can analyze hundreds to thousands of genes simultaneously, making it the foundation of expanded carrier screening (ECS). This advancement allows healthcare providers to screen for both common and rare genetic disorders in a single test, significantly improving clinical utility. The scalability of sequencing platforms and declining per-sample costs have made DNA sequencing an increasingly viable option for routine clinical use.
The growth of this segment is further supported by rising global awareness of inherited genetic disorders, a shift toward preventive healthcare, and broader integration of genomic testing into clinical guidelines. Advances in bioinformatics and artificial intelligence (AI) now enable faster interpretation of vast genomic datasets, reducing turnaround times and improving accuracy. These improvements have expanded the appeal of sequencing-based carrier screening beyond fertility clinics and hospitals to direct-to-consumer (DTC) models, where couples increasingly seek genetic insights prior to conception.
End Use Insights
Laboratories dominated the market in 2025, accounting for a 42.95% share, as laboratories are becoming one of the fastest-growing end use segments in the carrier screening market due to their specialized testing capabilities and access to advanced genetic technologies. Unlike hospitals that primarily focus on patient care, laboratories dedicate their infrastructure to processing large volumes of samples with high precision and efficiency. The growing demand for carrier screening tests for inherited disorders has led to an expansion of laboratory networks worldwide.
The laboratories segment is also benefiting from rising partnerships with healthcare providers, fertility clinics, and direct-to-consumer (DTC) genetic testing companies. Such collaborations allow laboratories to expand their testing reach while ensuring standardized and quality-controlled processes. Laboratories often serve as the backbone of large-scale carrier screening programs, handling both routine and specialized tests that require advanced expertise. Their ability to scale operations and process large numbers of genetic samples efficiently positions them as critical enablers of accessible carrier screening.

Moreover, the other end use segment in the carrier screening market includes entities such as fertility centers, research institutes, academic organizations, and direct-to-consumer (DTC) genetic testing companies. This diverse group is steadily gaining importance as demand for broader and more accessible genetic testing options increases. Fertility centers, in particular, are playing a growing role by integrating carrier screening into in vitro fertilization (IVF) and assisted reproductive technologies (ART) to minimize the risk of passing hereditary disorders to offspring. Research and academic institutions are contributing through large-scale studies and pilot programs that drive innovation and expand the scope of carrier screening panels.
Direct-to-consumer genetic testing companies represent another dynamic growth driver, as they offer affordable and convenient home-based testing kits, widening access for individuals who may not visit hospitals, laboratories, or clinics. Growing consumer awareness of reproductive health and technological advancements in sample collection and data interpretation are fueling adoption in this segment. Moreover, supportive government initiatives, coupled with collaborations between private companies and academic groups, are strengthening service availability. While currently smaller in scale compared to hospitals or laboratories, the “Other” segment is expected to expand rapidly, driven by accessibility, innovation, and its ability to reach underserved populations.
Regional Insights
The carrier screening market in North America is experiencing strong growth, due to leading role in the adoption of carrier screening, with the U.S. driving much of the growth due to advanced healthcare infrastructure and the integration of genetic testing into prenatal care. For instance, in May 2024, The Centers for Disease Control and Prevention (CDC) highlights that cystic fibrosis (CF) remains one of the most common inherited disorders, affecting about 35,000 people in the U.S., with one in every 31 Americans being a symptomless carrier of a CF gene mutation.
U.S. Carrier Screening Market Trends
The U.S. carrier screening market is growing fueled high prevalence has made CF one of the first conditions routinely included in carrier screening. Similarly, initiatives by the American College of Obstetricians and Gynecologists (ACOG) recommend pan-ethnic expanded carrier screening, enabling broader adoption across diverse populations.
Europe Carrier Screening Market Trends
Expanded carrier screening (ECS) is gradually gaining traction across Europe, particularly in private fertility clinics, while public healthcare systems remain cautious due to cost, equity, and resource considerations. Moreover, the European market is burgeoning due to factors such as rising awareness of genetic disorders, advancements in next-generation sequencing, increasing demand for personalized medicine, and supportive regulatory frameworks. France and Spain are also witnessing stronger patient and professional advocacy for expanded access to genetic testing. In France, targeted screening is available for couples with a family history of monogenic disorders, but patient support for wider access is clear. For instance, a 2025 Elsevier study found that over 90% of survey respondents considered preconception genetic carrier screening a major advancement, with nearly half supporting universal availability, thus driving the genetic carrier screening service market
The UK carrier screening market is growing rapidly. For instance, the Royal College of Obstetricians and Gynaecologists (RCOG) recognizes ECS as a valuable tool in reproductive medicine, but its NHS use is largely restricted to newborn screening through the heel prick test. Despite this, momentum is building, with policy discussions around integrating advanced approaches such as whole-genome sequencing (WGS). A key development came in September 2025, when Muscular Dystrophy UK reported that Scotland would add spinal muscular atrophy (SMA) to its NHS newborn screening panel starting in 2026, marking a significant step forward in rare disease detection.
Germany demonstrates a similar pattern, where private laboratories offer broad genetic testing panels, while public services remain selective. The German S2 Guideline for Human Genetics emphasizes the importance of carrier testing for individuals at risk, and national registries continue to highlight unmet needs. For instance, Mukoviszidose e.V. reported in its 2023 Cystic Fibrosis Registry that 7,587 people were living with cystic fibrosis, with widespread access to CFTR modulators improving median survival and lung function.
Asia Pacific Carrier Screening Market Trends
The Asia-Pacific carrier screening market is growing rapidly, driven by rising awareness of genetic disorders and rapid technological advancements. For instance, in February 2025, MedGenome launched the campaign “Care for the Rare” to promote early detection of rare diseases in Bengaluru, India. The campaign was unveiled with a documentary aimed at highlighting the critical role of genomic testing in the early detection and management of rare diseases. It emphasized the profound impact of undiagnosed inherited diseases on patients and honored families caring for loved ones affected by rare conditions. Therefore, such awareness is boosting the growth of the Asia Pacific market.
In Japan, private clinics and hospitals are increasingly offering expanded carrier screening (ECS) using next-generation sequencing (NGS) panels to identify couples at risk of passing on autosomal recessive and X-linked conditions. For instance, in June 2025, Cureus reported a case of a young Japanese man with CFTR dysfunction who developed severe lung disease and died at 32, underscoring the importance of early genetic risk identification and timely interventions.
China represents one of the fastest-growing markets for carrier screening due to its large population and high prevalence of carriers for hereditary conditions. Expanding access to cost-effective ECS panels has enabled broader adoption, particularly among couples planning for children. For example, in October 2024, Prenatal Diagnosis published a study showing that 65.87% of 2,168 healthy Chinese individuals carried at least one pathogenic variant, while 11.76% of couples were at risk, highlighting the need for population-specific carrier screening strategies.
Latin America Carrier Screening Market Trends
The Latin American carrier screening market is steadily advancing, fueled by rising awareness of genetic disorders and improvements in healthcare infrastructure. Countries across the region are recognizing the importance of early detection to reduce the burden of inherited diseases and improve patient outcomes. In Brazil, for example, the National Center for Biotechnology Information (NCBI) published a study in January 2023 analyzing 70 cystic fibrosis care centers (CFCCs), which revealed gaps in multidisciplinary teams and access to treatments like CFTR modulators, highlighting the need for broader carrier screening programs.
Middle East and Africa Carrier Screening Market Trends
The Middle East and Africa carrier screening market is experiencing strong growth, supported by rising awareness of genetic disorders, the high prevalence of inherited diseases, and increasing government-led initiatives to promote preventive healthcare. In South Africa, the South African Cystic Fibrosis Association (SACFA) reported that around 700 people were living with cystic fibrosis, though many in rural areas were misdiagnosed and lacked access to treatment, leading to complications such as malnutrition and pneumonia. This reality underscores the critical importance of expanding carrier screening to improve early detection and equitable access to care.
Key Carrier Screening Company Insights
The carrier screening market features several key players driving innovation and adoption. Leading companies include Myriad Genetics, Inc., BGI Genomics, Illumina, Inc., Thermo Fisher Scientific, Inc, F. Hoffmann-La Roche Ltd, Laboratory Corporation of America Holdings (LabCorp), Otogenetics Corporation, MedGenome, GeneTech (ATS Genetech Pvt Ltd), and CENTOGENE GmbH. These firms are heavily investing, rapidly evolving with continuous product innovation, geographic expansion, and strategic collaboration.
Key Carrier Screening Companies:
The following are the leading companies in the carrier screening market. These companies collectively hold the largest market share and dictate industry trends.
- Myriad Genetics, Inc.
- BGI Genomics
- Illumina, Inc.
- Thermo Fisher Scientific, Inc
- F. Hoffmann-La Roche Ltd
- Laboratory Corporation of America Holdings (LabCorp)
- Otogenetics Corporation
- MedGenome
- GeneTech (ATS Genetech Pvt Ltd)
- CENTOGENE GmbH
Recent Developments
-
In September 2025, PacBio announced that it is entering the high-throughput carrier screening market by expanding its PureTarget product portfolio. The new suite enables laboratories to consolidate multiple specialized tests into a single scalable assay using its HiFi sequencing technology, able to handle challenging genes associated with inherited conditions.
-
In August 2025, Natera introduced Fetal Focus, a non-invasive prenatal test (NIPT) intended to detect fetal mutations for several autosomal recessive single-gene disorders (including cystic fibrosis, spinal muscular atrophy, alpha-thalassemia, and beta-hemoglobinopathies) using a maternal blood sample, even when the biological father’s sample is unavailable.
-
In May 2025, CENTOGENE announced the launch of its new Reproductive Genetics Portfolio, introducing both Preimplantation Genetic Testing for Aneuploidy (PGT-A) and comprehensive carrier screening services. The PGT-A test, enhanced by CENTOGENE’s database of over one million sequences, offers high sensitivity, diagnostic accuracy, and an optional rapid turnaround of up to 24 hours for IVF embryos. Alongside this, the carrier screening service (CentoScreen) provides ≥99% coverage of 332 genes, available for individuals or couples, enabling early detection of inherited genetic risks.
-
In October 2024, Blueprint Genetics introduced 6 new reproductive carrier screening tests that include FMR1 repeat expansion analysis. These are various screens (Comprehensive, Core, Ashkenazi Jewish, and versions for couples (“DUO”)) now upgraded with FMR1 repeat expansion capability.
Carrier Screening Market Report Scope
Report Attribute
Details
Market size value in 2026
USD 1.77 billion
Revenue forecast in 2033
USD 4.05 billion
Growth rate
CAGR of 12.55% from 2026 to 2033
Actual data
2021 - 2025
Forecast period
2026 - 2033
Quantitative units
Revenue in USD billion/million and CAGR from 2026 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Medical conditions, type, technology, end use, and region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Norway; Sweden; Denmark; Japan; China; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Myriad Genetics, Inc.; BGI Genomics; Illumina, Inc.; Thermo Fisher Scientific, Inc; F. Hoffmann-La Roche Ltd; Laboratory Corporation of America Holdings (LabCorp); Otogenetics Corporation; MedGenome; GeneTech (ATS Genetech Pvt Ltd); CENTOGENE GmbH
Customization scope
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Carrier Screening Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the global carrier screening market based on medical conditions, type, technology, end use, and region:
-
Medical Conditions Outlook (Revenue, USD Million, 2021 - 2033)
-
Spinal Muscular Atrophy
-
Cystic Fibrosis
-
Tay-Sachs
-
Gaucher Disease
-
Sickle Cell Disease
-
Others
-
-
Type Outlook (Revenue, USD Million, 2021 - 2033)
-
Expanded Carrier Screening
-
Targeted Disease Carrier Screening
-
-
Technology Outlook (Revenue, USD Million, 2021 - 2033)
-
DNA Sequencing
-
Polymerase Chain Reaction
-
Microarrays
-
Other Technologies (MLPA, Genotyping, Enzyme Screening)
-
-
End-use Outlook (Revenue, USD Million, 2021 - 2033)
-
Hospitals
-
Laboratories
-
Physician Offices & Clinics
-
Other End Users
-
-
Regional Outlook (Revenue, USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global carrier screening market size was estimated at USD 1.64 billion in 2025 and is expected to reach USD 1.77 billion in 2026.
b. The global carrier screening market is expected to grow at a compound annual growth rate of 12.55% from 2026 to 2033 to reach USD 4.05 billion by 2033.
b. North America dominated the carrier screening market with a share of 46.73% in 2025. This is attributable to leading role in the adoption of carrier screening, with the U.S. driving much of the growth due to advanced healthcare infrastructure and the integration of genetic testing into prenatal care
b. Some key players operating in the carrier screening market include Myriad Genetics, Inc., BGI Genomics, Illumina, Inc., Thermo Fisher Scientific, Inc, F. Hoffmann-La Roche Ltd, Laboratory Corporation of America Holdings (LabCorp), Otogenetics Corporation, MedGenome, GeneTech (ATS Genetech Pvt Ltd), and CENTOGENE GmbH
b. Key factors that are driving the market growth include rising prevalence of genetic disorders, technological advancements in genetic testing, growing awareness and demand for personalized medicine
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










