- Home
- »
- Medical Devices
- »
-
Clot Management Devices Market Size & Share Report, 2030GVR Report cover
![Clot Management Devices Market Size, Share & Trends Report]()
Clot Management Devices Market (2023 - 2030) Size, Share & Trends Analysis Report By Product (Neurovascular Embolectomy Devices, Percutaneous Thrombectomy Devices), By End-use (Diagnostic Centers, Hospitals), By Region, And Segment Forecasts
- Report ID: GVR-1-68038-201-3
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Report Overview
The global clot management devices market size was valued at USD 1.72 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 5.0% from 2023 to 2030. Clot management devices are medical tools used to treat and prevent blood coagulum that can lead to serious health complications. They target blood coagulum in arteries or veins, reducing the risk of heart attacks, strokes, or deep vein thrombosis. The market is poised for significant growth, fueled by the increasing demand for effective thrombectomy/embolectomy devices and a growing awareness of newer approaches to clot management.
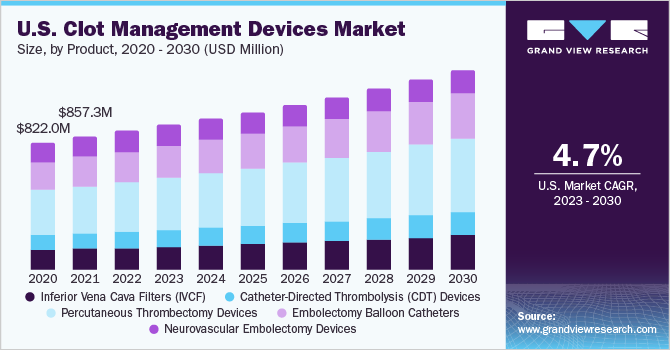
Venous thromboembolism, including pulmonary embolism and deep vein thrombosis, has a global impact that surpasses AIDS, cancer, and road traffic accidents combined, underlining the need for comprehensive clot management solutions.Stroke ranks among the top causes of disabilities and is the fifth leading cause of death in the U.S., as per data published by the CDC. Additionally, the CDC estimates that almost 900,000 people in the U.S. are affected by pulmonary embolism (PE) and deep vein thrombosis (DVT). Factors such as the increasing prevalence of cardiovascular diseases, an aging population, and advancements in medical technology further contribute to the growing market demand.
The rising global incidence of cardiovascular diseases, leading to a significant number of deaths each year, has driven the demand for clot management devices. As per the World Health Organization, cardiovascular diseases are the leading global cause of death, responsible for around 17.9 million deaths annually. As the population ages, the risk factors associated with blood coagulum formation also rise, emphasizing the need for effective solutions. Healthcare providers are actively seeking advanced clot management devices to address the growing burden of CVDs and mitigate the risks associated with blood coagulum.
Furthermore, technological advancements in clot management devices have fueled market growth. Innovative devices such as catheters, filters, and stents are being developed to provide better coagulum retrieval and dissolution capabilities. For instance, newer-generation clot retrieval devices, known as mechanical thrombectomy devices, have gained popularity in recent years.
For instance, in January 2023, Penumbra, Inc. introduced Lightning Flash, an advanced mechanical thrombectomy system with dual clot detection algorithms, following FDA clearance, making it the most powerful and innovative device in the market. The continuous development of such cutting-edge technologies has resulted in increased adoption rates and contributed to the growth of the market.
The COVID-19 pandemic has had a significant impact on the market, as it has emphasized the importance of effective clot management in critically ill patients. COVID-19 has been associated with an increased risk of blood coagulum, leading to a heightened awareness of clot management and the need for appropriate devices. Healthcare providers have utilized clot retrieval devices in treating COVID-19 patients with severe lung involvement and blood coagulum-related complications, improving patient outcomes. The pandemic is expected to have a lasting impact on clot management devices, as healthcare systems prioritize the prevention and management of coagulum-related complications in diverse patient populations.
Product Insights
The percutaneous thrombectomy devices segment dominated the market with a 36.0% revenue share in 2022. The dominance of the percutaneous thrombectomy device segment in the market can be attributed to its distinct advantages over traditional invasive procedures. Percutaneous interventions, performed through small incisions and guided by imaging techniques, offer shorter recovery times and reduced complications, appealing to both patients and healthcare providers. In conditions like Deep Vein Thrombosis (DVT) and arterial thrombosis, percutaneous medical devices play a crucial role in restoring blood flow.
Thrombectomy devices, along with aspiration catheters and other systems, form effective percutaneous thrombectomy systems, providing successful clot management with high rates of success and minimal adverse events. The combination of these less invasive procedures and the efficacy of percutaneous medical devices has established their dominance, enabling improved patient outcomes and a growing preference for them in cardiovascular treatments. Based on product, the clot management devices market is classified into neurovascular embolectomy device, embolectomy balloon catheters, percutaneous thrombectomy device, catheter-directed thrombolysis (CDT) device, and inferior vena cava filters (IVCF).
The neurovascular embolectomy devices segment is anticipated to witness the highest CAGR of 6.1% during the forecast period, which can be attributed to several key factors. These devices are specifically designed to treat medical conditions such as embolism, primarily occurring in brain blood vessels. Ischemic stroke, which accounts for 87% of all stroke cases globally, and thromboembolism are among the conditions treated by these devices. With the obstruction of blood flow resulting from cerebral thrombosis and cerebral embolism, the demand for these devices, particularly wire-caged devices like stent retrievers, for the mechanical treatment of ischemic stroke is increasing.
Furthermore, the incidence rate of thromboembolism is significant, with an estimated 250,000 cases annually in the U.S. whites alone. Given the high hospitalization rates and mortality associated with thromboembolic diseases, the use of clot management devices such as aspiration catheters, stent retrievers, balloon catheters, and distal protection devices has gained prominence in the treatment of thromboembolism.
End-use Insights
The hospitals segment dominated the market with a share of 80.2% in 2022. The dominance of the segment is driven by hospitals serving as the primary point of contact for patients with conditions like DVT or PE, recommending and utilizing these devices. Hospital committees and teams focus on selecting devices based on safety, efficacy, and cost, collaborating with value chain entities for access. Specialized departments, advanced labs, and operating rooms in hospitals provide an ideal setting for clot management procedures, meeting the increasing demand for cardiovascular treatments.
The negotiating power of hospitals with insurance companies and payers helps secure higher reimbursement rates, while strategic partnerships with medical technology firms enhance their ability to meet patient needs. The rise of telestroke services further supports the adoption of clot management devices, saving time in stroke treatment through remote connectivity. On the basis of end-use, the market for clot management devices is classified into diagnostic centers and hospitals.
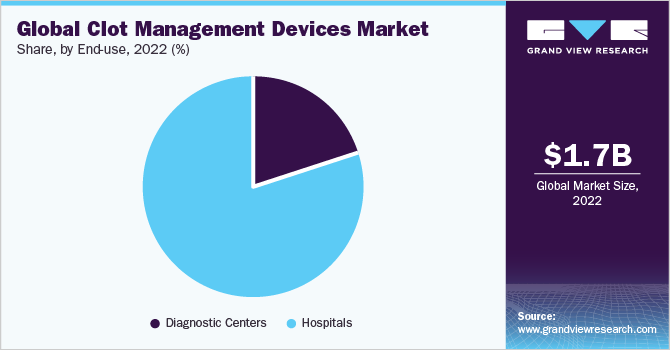
The diagnostic centers segment is anticipated to witness the highest CAGR of 5.4% during the forecast period. The strong growth is due to its crucial role in supporting clot management processes. By utilizing advanced imaging technologies like ultrasound and CTPA scans, diagnostic centers accurately determine coagulum positions, enabling effective clot management strategies and device selection. Moreover, diagnostic centers play a significant role in identifying cases of coagulum-related conditions such as DVT or PE, driving the demand for clot management devices.
The integration of AI in diagnostic centers further enhances their capabilities in detecting conditions such as carotid artery diseases on duplex ultrasound. Collaborations between diagnostic centers and healthcare providers facilitate personalized treatment plans and aid in selecting the most appropriate clot management device, based on clot characteristics, thus streamlining purchasing decisions for hospitals and contributing to the overall market growth.
Regional Insights
North America dominated the global market for clot management devices and accounted for 59.73% of the total revenue share in 2022. The growth of the market is attributed to the impact of the geriatric population in developed economies like the U.S. and Canada. With a notable increase in Venous Thromboembolism (VTE) incidences after the age of 60, the geriatric population faces a significantly higher risk of clot development, making them a key driver of market demand. Furthermore, the growing prevalence of high blood pressure and cholesterol among adults contributes to the regional market's dominance.
High blood pressure damages artery walls, increasing susceptibility to plaque deposits and clot formation, affecting approximately 122 million adults in the U.S., with only a quarter having hypertension under control. Similarly, high cholesterol levels, affecting 25 million adults with levels above 240 mg/dl, contribute to narrowed arteries and heightened demand for clot management devices. Together, the growing geriatric population and the rising prevalence of high blood pressure and cholesterol underscore the market dominance of clot management devices.
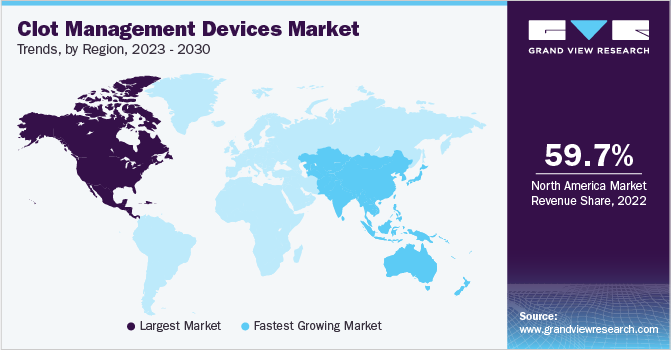
On the other hand, the Asia-Pacific region is expected to witness the fastest CAGR of 6.1% over the forecast period. The promising growth is due to the high prevalence of cardiovascular diseases in countries such as China, India, and Japan, which contributes to the increasing demand for cardiology medical devices for diagnosis and treatment. The substantial burden of CVDs and related mortalities highlights the need for advanced technologies in this region. Furthermore, companies in China, India, and Japan are actively developing and introducing innovative solutions to address the rising healthcare burden, driving the growth of the Asia Pacific market.
Key Companies & Market Share Insights
Market players are introducing advanced products at affordable prices to increase their market share. Key players are implementing strategic initiatives, such as mergers, acquisitions, and collaborations, to maximize their market dominance. For instance, in January 2023, Penumbra introduced its latest innovation in mechanical thrombectomy, called Lightning Flash. This advanced system has been cleared by the U.S. Food and Drug Administration (FDA) and is considered the most innovative and powerful mechanical thrombectomy device available in the market. Some of the notable players involved in the global clot management devices market include:
-
Medtronic
-
Boston Scientific Corporation
-
iVascular SLU
-
Teleflex Inc
-
Edwards Lifesciences Corporation
-
LeMaitre Vascular, Inc
-
Vascular Solutions, Inc
-
Straub Medical AG
-
Cook Medical
-
DePuy Synthes
Clot Management Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 1.79 billion
Revenue forecast in 2030
USD 2.53 billion
Growth rate
CAGR of 5.0% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
September 2023
Quantitative units
Revenue in USD million/billion, and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; South Korea; Australia; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Medtronic; Boston Scientific Corporation; iVascular SLU; Teleflex Inc.; Edwards Lifesciences Corporation; LeMaitre Vascular, Inc.; Vascular Solutions, Inc.; Straub Medical AG; Cook Medical; DePuy Synthes
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to Country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Clot Management Devices Market Report Segmentation
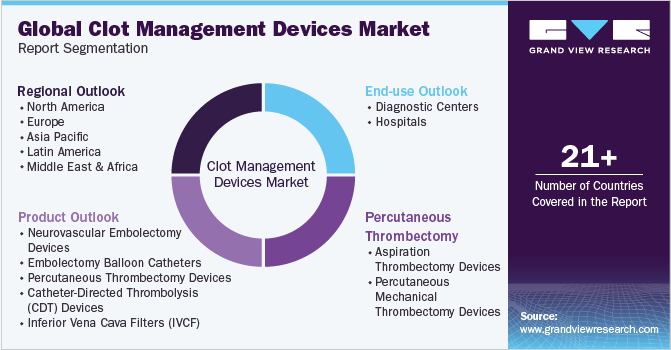
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global clot management devices marketreport based on product, end-use, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Neurovascular Embolectomy Devices
-
Embolectomy Balloon Catheters
-
Percutaneous Thrombectomy Devices
-
Catheter-Directed Thrombolysis (CDT) devices
-
Inferior vena cava filters (IVCF)
-
-
Percutaneous Thrombectomy Devices Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Aspiration Thrombectomy Devices
-
Percutaneous Mechanical Thrombectomy Devices
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Diagnostic centers
-
Hospitals
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Norway
-
Denmark
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global clot management devices market size was estimated at USD 1.72 billion in 2022 and is expected to reach USD 1.79 billion in 2023.
b. The global clot management devices market is expected to grow at a compound annual growth rate of 5.0% from 2023 to 2030 to reach USD 2.53 billion by 2030.
b. North America dominated the clot management devices market with a share of 59.7% in 2022. This is attributable to high prevalence of cardiovascular and peripheral diseases, growing geriatric population, presence of a well-developed reimbursement framework.
b. Some key players operating in the clot management devices market include Medtronic; Boston Scientific Corporation; iVascular; Teleflex; Edwards Lifesciences; LeMaitre Vascular; Vascular Solutions, Inc.; and Straub Medical.
b. Key factors that are driving the market growth include increasing prevalence of stroke, Pulmonary Embolism (PE), Deep Vein Thrombosis (DVT), obesity, technological advancements, and improvement in health insurance coverage & availability of emergency medical services.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










