- Home
- »
- Medical Devices
- »
-
Controlled Release Drug Delivery Market Size Report, 2033GVR Report cover
![Controlled Release Drug Delivery Market Size, Share & Trends Report]()
Controlled Release Drug Delivery Market (2025 - 2033) Size, Share & Trends Analysis Report By Technology (Targeted Delivery, Transdermal), By Release Mechanism, By Application (Metered Dose Inhalers, Injectable), By Region, And Segment Forecasts
- Report ID: GVR-2-68038-347-8
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2021 - 2023
- Forecast Period: 2025 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Controlled Release Drug Delivery Market Summary
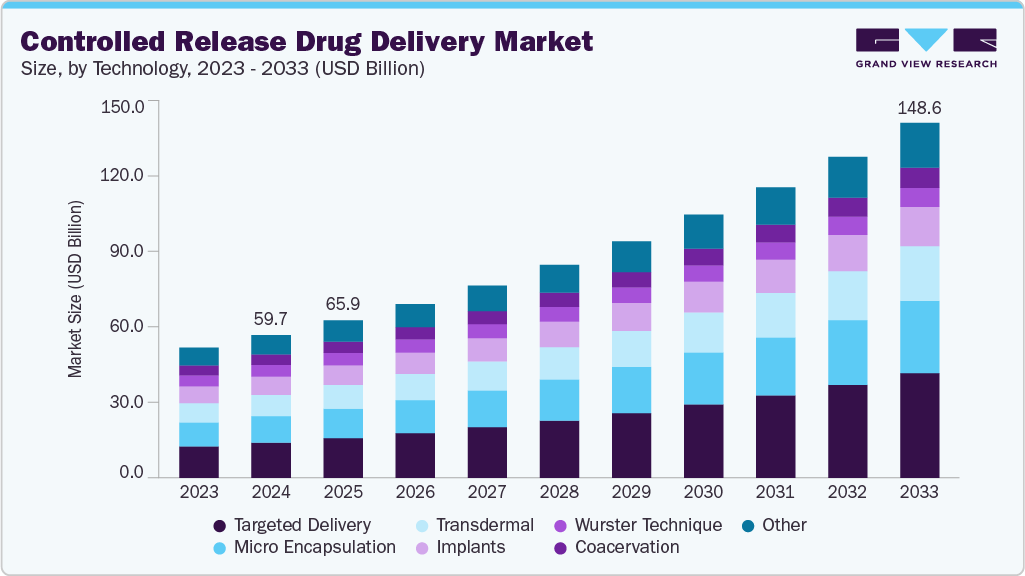
The global controlled release drug delivery market size was estimated at USD 59.8 billion in 2024 and is projected to reach USD 148.6 billion by 2033, growing at a CAGR of 10.7% from 2025 to 2033. Changing prescription patterns of physicians preferring controlled-release drug delivery over conventional systems, owing to the benefits such as high therapeutic efficacy, better patient compliance, and reduced treatment cost, is expected to contribute to the market's growth over the forecast period.
Key Market Trends & Insights
- North America dominated the controlled release drug delivery market with the largest revenue share of 42.6% in 2024.
- The controlled release drug delivery market in the Asia Pacific accounted for the largest market revenue share in 2024.
- By technology, the targeted delivery segment led the market with the largest revenue share of 24.7% in 2024.
- By release mechanism, the feedback-regulated drug delivery systems segment accounted for the largest market revenue share in 2024.
- By application, the oral controlled drug delivery systems segment accounted for the largest market revenue share in 2024.
Market Size & Forecast
- 2024 Market Size: USD 59.8 Billion
- 2033 Projected Market Size: USD 148.6 Billion
- CAGR (2025-2030): 10.7%
- North America: Largest market in 2024
Moreover, being a part of a highly competitive and fragmented market, pharmaceutical companies are proactive with the changing market requirements and continuously invest in developing controlled-release drug delivery systems, thereby significantly driving the demand.Constantly rising R&D expenditure by pharmaceutical development companies focused on developing efficient therapy for chronic and non-communicable complications such as cancer, diabetes, and hypertension is expected to boost the market growth over the forecast period. For instance, Major pharmaceutical companies such as AbbVie, AstraZeneca, and Merck are implementing controlled drug release systems to enhance the treatment of chronic diseases, cancer, and neurological disorders.
The rising global geriatric and pediatric population is one of the major factors contributing to market growth, largely due to non-adherence to the medicine regimen, which is very common among these age groups. Geriatric populations suffer from compromised physical, mental, and biological functions that affect their medication intake. Also, their bodies cannot tolerate high doses and harsh drug side effects. Thus, the demand for controlled-release drug delivery systems is expected to grow with a steeply rising patient population.
Constantly rising R&D expenditure by pharmaceutical development companies focused on developing efficient therapies for chronic and non-communicable complications such as cancer, diabetes, and hypertension is expected to boost the market growth over the forecast period. For instance, Major pharmaceutical companies such as AbbVie, AstraZeneca, and Merck are implementing controlled drug release systems to enhance the treatment of chronic diseases, cancer, and neurological disorders.
The rising geriatric and pediatric population globally is one of the major factors contributing to market growth, largely due to non-adherence to the medicine regimen, which is very common among these age groups. Geriatric populations suffer from compromised physical, mental, and biological functions that affect their medication intake. Also, their bodies cannot tolerate high doses and the harsh side effects of drugs. Thus, the demand for controlled-release drug delivery systems is expected to grow with a steeply rising patient population.
Controlled release drug delivery significantly reduces dose and dosage frequency, prevents abnormal fluctuations of plasma drug levels, improves efficacy, enhances patient compliance, and achieves a uniform drug effect of the administered drug. Compared to traditional delivery systems, the controlled-release drug delivery systems achieve a prolonged therapeutic effect, delivering the drug at the target site at a pre-determined rate and with predictable drug release kinetics. Thus, with several added benefits offered by the controlled release delivery system, their adoption is expected to increase notably over the forecast period.
Market Concentration & Characteristics
The controlled-release drug delivery systems industry is fast-growing due to advances in formulation technology and new-generation delivery platforms. AstraZeneca points out that research in advanced delivery technologies, such as lipid nanoparticles and microsphere-based systems, is intended to enhance dosing convenience, accuracy, and therapeutic impact. ScienceDirect indicates that drug-loaded microspheres form a central method in controlled release systems where therapeutics can be delivered sustainably and selectively. The sector is dominated by a mix of big pharma companies, including AstraZeneca, driving innovation and adoption of technology, with specialized research-oriented firms investigating microsphere and nanoparticle-based platforms. These blends promote a competitive landscape where technology evolution and R&D strength are key differentiators across industry players.
Progress in controlled-release drug delivery systems enhances therapeutics through technologies like polymeric nanoparticles, liposomes, and hydrogels, enabling targeted and sustained medication release. GSC Biological and Pharmaceutical Sciences highlights increased therapeutic effectiveness and reduced side effects, while PolimerBio emphasizes improved solubility, targeted delivery, and patient adherence. These developments reflect significant innovation, pushing drug delivery towards more precise, efficient, and patient-centered treatments.
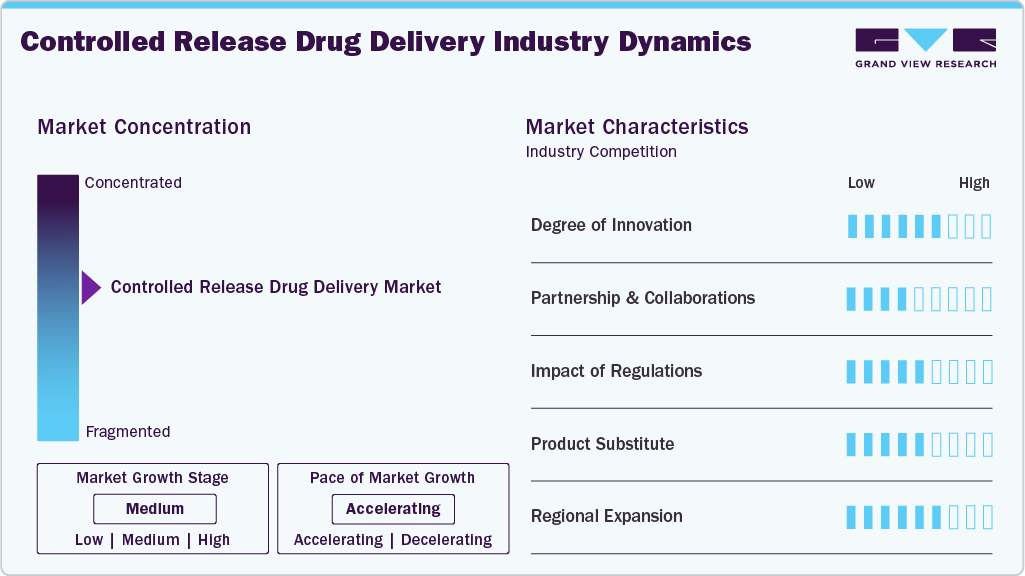
Partnerships and collaborations drive innovation in controlled drug delivery systems by enabling access to advanced technologies and improving therapeutic outcomes. For example, in September 2024, Nanoform Finland and Celanese Corporation combined Nanoform’s CESS nanoparticle platform with Celanese’s VitalDose implant technology to develop long-acting biologic implants, including a patient-centric solution for multiple sclerosis. Such strategic collaborations enhance precision, sustained drug release, and treatment adherence, reflecting how partnerships accelerate innovation and expand capabilities in the drug delivery sector.
Controlled-release drug delivery systems are tightly regulated to ensure safety, efficacy, and quality. In the U.S., the FDA requires pre-clinical studies, IND applications, clinical trials, and NDA submissions, with expedited pathways for drugs addressing serious conditions. The MHRA oversees approvals through MAAs and CTAs in the UK, ensuring GMP compliance and consistent drug release. These frameworks safeguard patients and uphold high standards in global pharmaceutical practice.
Long-acting injectable drugs are an effective alternative to currently available in The controlled-release drug delivery systems industry. For example, Novartis's new product, Leqvio, provides sustained therapeutic effects with fewer doses, promoting adherence and improved clinical outcomes. Smart injection devices with IoT connectivity can provide real-time monitoring of patients and personalized therapy for chronic conditions. Long-acting injectable medications can provide a practical patient-centered alternative to traditional controlled-release drug formulations while maintaining drug efficacy.
Growth in the The controlled-release drug delivery systems industry is bolstered by regional expansion, enhancing access to advanced and patient-friendly therapies. The UK market is advancing through technological innovations and personalized medicine, meeting the rising need for treatment regimens that improve adherence and outcomes. North America leads due to a robust pharmaceutical R&D infrastructure, an increasing geriatric population, and significant investments by key market players to expand manufacturing capabilities, supply chains, and regional presence. Strategic initiatives and investments across these regions continue to drive market growth and adoption of advanced drug delivery systems.
Technology Insights
The targeted delivery segment led the market with the largest revenue share of 24.7% in 2024, as many market participants have a strong portfolio of technology. Targeted delivery offers the preferred site of action and can help move drugs away from sites that might lead to drug toxicity. Since these systems provide increased bioavailability, targeted technologies provide localization of the drug, which leads to improved absorption, reduced fluctuation in circulating drug levels, and a low risk of side effects. It’s a commonly preferred drug delivery technology.
The micro encapsulation segment is anticipated to witness at the fastest CAGR of 11.8% during the forecast period. The rising demand to improve the shelf-life and achieve controlled drug release of highly complex and unstable molecules such as proteins, vitamins, and antioxidants is expected to foster the growth of the microencapsulation segment over the forecast period. For instance, vitamin A is characterized by poor water solubility and chemical stability, but when microencapsulated, the product's shelf life increases, concomitantly helping achieve controlled drug release.
Release Mechanism Insights
The feedback-regulated drug delivery systems segment led the market with the largest revenue share of 27.3% in 2024, owing to their therapeutic efficiency in treating disorders such as diabetes. In addition, increasing R&D activities to exploit the potential of feedback-regulated controlled drug delivery systems as antidotes is expected to aid market growth. These systems offer exceptional precision, reducing the risk of over- or under-dosing, and are especially valuable in managing chronic conditions like diabetes, hormonal disorders, or cancer. Their ability to provide closed-loop control increases patient safety, compliance, and therapeutic effectiveness.
The activated modulated drug delivery system segment is anticipated to witness at the fastest CAGR of 11.1% during the forecast period. The activated modulated drug delivery system represents a substantial advancement in controlled release technology as it allows for very precise modulation of drug delivery via multiple mechanisms. Unlike traditional systems that only utilize controlled release via passive means, AMDDS can activate and modulate drug delivery using physical, chemical, and biological processes and external energy sources. This way, drug delivery is customized to specific therapeutic use and maximizes efficacy while minimizing potential side effects. Activation and modulation through different mechanisms enhance the precision and responsiveness of drug delivery, thus moving pharmaceutical delivery systems forward with portability and interdisciplinary approaches.
Application Insights
The oral controlled segment led the market with the largest revenue share of 35.3% in 2024, owing to its frequent usage and availabilityThe oral controlled drug delivery system market is growing enormously on account of factors like the capacity for drug release at a fixed rate, sustaining maximum therapeutic levels for prolonged durations, and reducing plasma drug concentration fluctuations. This controlled release improves bioavailability, lowers the frequency of dosing, and enhances patient compliance, especially in the case of chronic diseases. Emerging new formulation technologies and biomaterials have made it possible for targeted delivery, whereby the drugs can work more effectively at localized sites with diminished systemic side effects. Further, developments in polymer-based and biodegradable delivery systems are broadening applications of CDDS in all potential therapeutic areas, making it one of the fastest-growing segments of the pharmaceutical drug delivery market.
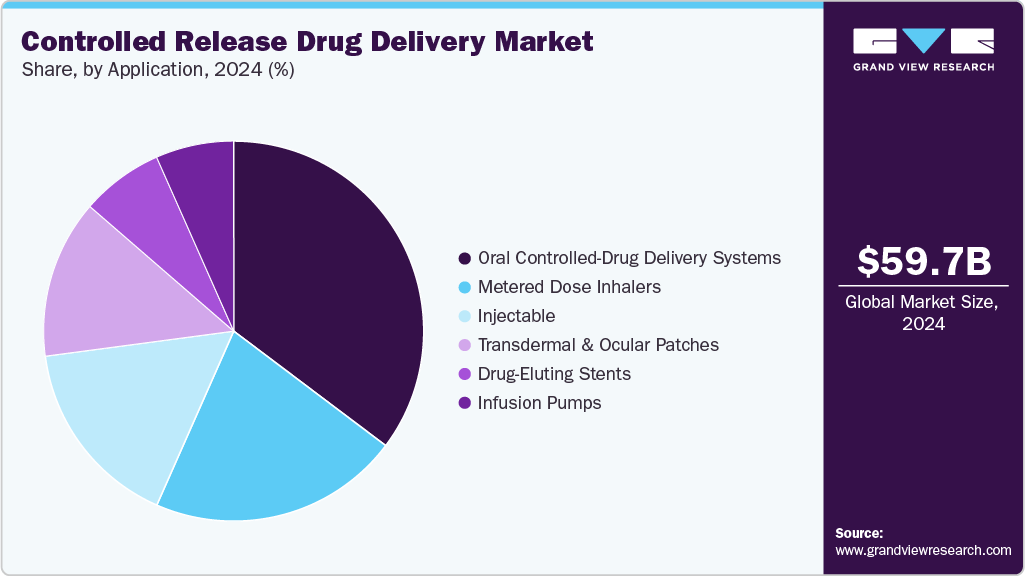
The injectables segment is expected to grow at the fastest CAGR of 12.2 % during the forecast period, owing to a wide product diversity and usage in treating various diseases. Long-acting injectables are preferred to conventional delivery forms as they offer several advantages, including a predictable drug release profile during a definite period, enhanced patient compliance, improved systemic availability of drugs, ease of application, and thus an overall reduction in medical costs.
Regional Insights
North America dominated the controlled release drug delivery market with the largest revenue share of 42.63% in 2024, is driven by the rising prevalence of various chronic diseases, which necessitate more effective and patient-friendly treatment options. Furthermore, the rising geriatric population in North America contributes to market expansion, as older adults often require multiple medications and benefit from the convenience and reduced dosing frequency offered by these technologies. According to the National Council on Aging (NCOA), the elderly population in the U.S. aged 65 years and above is expected to increase from 57.8 million in 2022 to around 78.3 million in 2040.
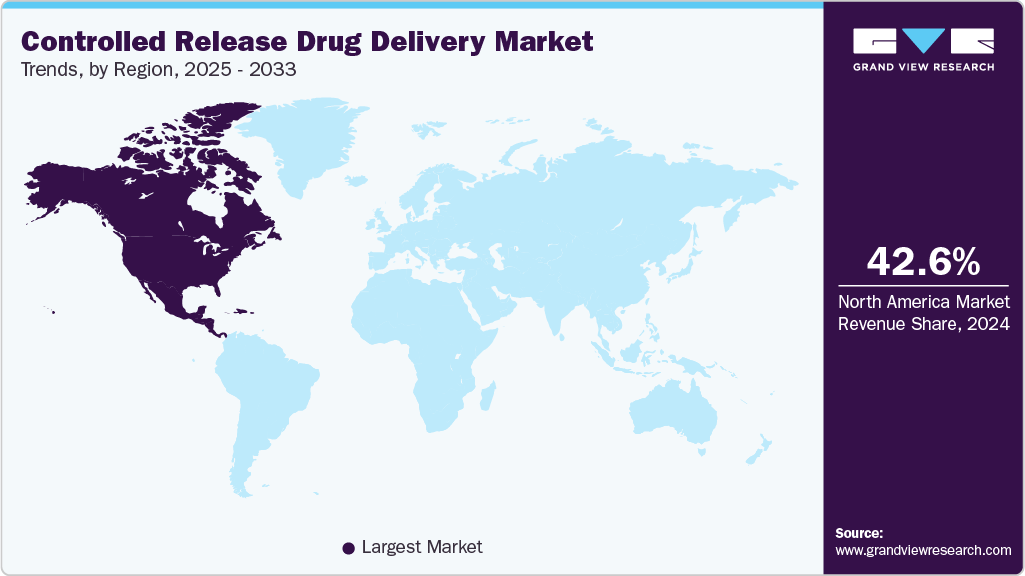
Similarly, according to Statistics Canada, in July 2023, around 7.6 million Canadians, or approximately 18.9% of the country’s population, were aged 65 years and above, which is expected to reach 21.4% to 23.4% by 2030. This aging population, coupled with robust pharmaceutical research and development (R&D) infrastructure in North America and significant investments in advanced drug delivery systems, is expected to drive the market growth in the region.
U.S. Controlled release drug delivery market Trends
The controlled release drug delivery market in the U.S. is driven by the increasing prevalence of chronic diseases, advancements in drug delivery technologies, and the growing demand for improved patient outcomes and adherence. The benefits of controlled-release systems, such as reduced dosing frequency, minimized side effects, and enhanced drug efficacy, have fueled their adoption across various therapeutic areas in the country. Furthermore, the rising investments in research and development by pharmaceutical companies and the increasing number of product approvals by regulatory bodies such as the FDA have contributed to the market's expansion.
IFF’s new controlled release platform, launched in May 2024, directly supports advancements in drug delivery by offering innovative excipients such as METHOCEL, ETHOCEL, and POLYOX, which are widely used in sustained and controlled-release formulations. The platform enhances formulation efficiency, patient compliance, and timed drug release, aligning with key market demands in the U.S. for chronic disease management and personalized medicine.
Europe Controlled Release Drug Delivery Market Trends
The controlled release drug delivery market in Europe is witnessing steady growth, driven by rising chronic disease prevalence, technological innovation, and increasing patient preference for convenient, long-acting therapies. Chronic conditions such as diabetes, cardiovascular disorders, and cancer are prevalent across Europe, necessitating advanced drug delivery solutions that improve treatment efficacy and reduce dosing frequency. The growing diabetes burden across Europe is significantly driving the demand for controlled-release drug delivery systems.
The UK controlled release drug delivery market is evolving rapidly, propelled by technological innovations, advances in personalized medicine, and an increasing demand for treatment regimens that support better patient adherence and outcomes. With the growing burden of chronic diseases, the need for simplified and effective drug delivery systems is more urgent than ever. As a professor from the University of Nottingham’s School of Pharmacy highlighted,
“The future of prescribed medication lies in a personalized approach, and we know that up 50% of people in the UK alone don’t take their medicines correctly and this has an impact on poorer health outcomes with conditions not being controlled or properly treated. A single pill approach would simplify taking multiple medications at different times and this research is an exciting step towards that.”
This underscores the importance of controlled-release technologies in addressing non-adherence and improving therapeutic success.
The controlled release drug delivery market in Germany is poised for significant growth, fueled by its aging population, strong pharmaceutical infrastructure, and continued innovation in drug delivery technologies. Germany is witnessing a demographic shift as one of only five “super-aged” societies globally. The population aged 65 and older is projected to grow by 41%, reaching 24 million by 2050, or nearly one-third of the country’s total population. This trend creates increased demand for drug delivery systems that offer improved compliance, reduced dosing frequency, and better therapeutic outcomes for elderly patients managing chronic diseases.
Asia Pacific Controlled release drug delivery market Trends
The controlled release drug delivery market in Asia Pacific is driven by developing healthcare infrastructure, growing investments in pharmaceutical R&D, and rising demand for high-compliance therapies. Countries such as Japan, South Korea, China, and India are witnessing significant demand owing to the growing burden of chronic conditions such as diabetes, cardiovascular diseases, and cancer, which require long-term and consistent treatment regimens. Pharmaceutical companies are increasingly adopting controlled-release formulations to improve drug bioavailability and reduce dosing frequency. Furthermore, local governments are encouraging domestic manufacturing through favorable drug regulatory reforms and incentives for innovation.
The China controlled release drug delivery market is driven by the aging population and the increasing prevalence of chronic diseases, such as diabetes, cardiovascular diseases, and cancer. According to a report published by the Ministry of Civil Affairs and China National Committee, China's population aged 60+ reached 297 million, 21.1% of the total, with those aged 65+ at 216.76 million (15.4%). The rising prevalence of diseases in the country often requires long-term medication, making controlled-release drug delivery systems highly preferred for improved patient compliance and therapeutic efficacy. According to the International Diabetes Federation, around 147.98 million people, or 11.9% of the population, had diabetes in 2024. Such high prevalence of diseases, coupled with the Chinese government's focus on healthcare reform and local manufacturing, is expected to drive the market growth.
The controlled release drug delivery market in Japan is witnessing significant growth, driven by the country's aging population, increasing prevalence of chronic diseases, and strong government initiatives supporting advancements in the country’s healthcare industry. Japan’s elderly population reached a record 36.25 million, with those aged 65 or older comprising nearly one-third (29.3%) of the total population, according to government data from the Ministry of Internal Affairs and Communications in September 2024. This is the highest proportion globally among regions with over 100,000 residents. This high proportion of elderly population increases the demand for advanced drug delivery technologies in the country that ensure precise and controlled medication administration, particularly for managing conditions such as diabetes, respiratory disorders, and cardiovascular diseases.
The India controlled release drug delivery market is experiencing significant expansion, driven by the increasing prevalence of chronic diseases, advances in technology, the evolving healthcare industry, and the increasing prevalence of diabetes and asthma. A report from the WHO regarding disease prevalence in India published in March 2023 estimated that by 2025, around 75 million people in India will be receiving treatment for either diabetes or hypertension, with approximately one in four adults being hypertensive and one in ten adults being diabetic. Likewise, around 35 million people in India have asthma, according to the Global Asthma Report 2022. The prevalence of various diseases naturally increases the need for various advanced drug-delivery technologies in the country.
Latin America Controlled release drug delivery market Trends
The controlled release drug delivery market in Latin America is driven by the rising prevalence of chronic diseases such as diabetes, cardiovascular conditions, and cancer, which necessitate long-term medication management. According to the International Agency for Research on Cancer, the 5-year prevalence of cancer in Latin America and the Caribbean region was estimated to be around 4.1 million in 2022. The region's aging population further fuels this growth, as older individuals often require multiple medications, increasing the demand for formulations that improve patient adherence and reduce dosing frequency. Furthermore, increasing awareness about the benefits of these systems, such as enhanced drug efficacy, reduced side effects, and improved patient compliance. Latin America’s regulatory environment is becoming more receptive to advanced formulations, which is positively impacting the market.
The Brazil controlled release drug delivery market is driven by the rising occurrence of chronic disorders like diabetes, heart conditions, and cancer, which require extended medication and the aging population. For example, the census of 2022 brought to light that the number of elderly (60 and older) individuals in Brazil had increased by 57.4% in 12 years, bringing roughly 38 million elderly people. This has led to a growing need for drug delivery systems that could release medications substantively, increase patient compliance and minimize side effects.
Middle East & Africa Controlled release drug delivery market Trends
The controlled release drug delivery market in Middle East & Africa is growing rapidly. The region's economic growth, particularly in countries with high healthcare spending, creates a favorable environment for market expansion. Increased disposable incomes and improved access to healthcare services enable patients to afford advanced medications, including controlled-release formulations. The rising prevalence of lifestyle-related diseases, such as obesity and hypertension, further drives the demand for these drug delivery systems in the MEA. In addition, government initiatives to improve healthcare infrastructure and promote pharmaceutical innovation contribute to market growth. The development of local pharmaceutical industries and the increasing focus on generic drug manufacturing also play a crucial role in making controlled-release products more accessible and affordable, thereby expanding the market reach in the region.
The Saudi Arabia controlled release drug delivery market is driven by advanced healthcare infrastructure and technological advancements. The country's healthcare system is investing in advanced medical technologies and infrastructural improvements to tackle these health challenges, thus creating a conducive environment for the adoption of controlled-release drug delivery systems. The government's Vision 2030 initiative aimed at diversifying the economy and improving services in healthcare will also play a role in attracting foreign investment and stimulating innovation in the pharmaceutical sector. The awareness of the advantages of controlled-release formulations among healthcare professionals and patients is also increasing the market growth. Among the advantages of controlled-release formulations are the improved therapeutic effect, reduced adverse effects, and increased patient adherence.
The controlled release drug delivery market in Kuwait is driven by growing awareness of the advantages of controlled-release drug delivery technologies, growing disease prevalence, and growing investment in the country's healthcare infrastructure. The State of Kuwait is making considerable investments in healthcare infrastructure and new technologies, including robotic surgeries and development of new specialized centers, to relieve patients from having to travel abroad for care. This change is intended to lower overseas spending by providing hospitals in Kuwait, such as Adan and Sheikh Jaber hospitals, the capability to manage complex cases for medical conditions such as cancer and heart diseases. The main goal is to strengthen local services, so patients no longer require referrals to overseas care, therefore improving treatment coordination, and making Kuwait a regional center for medical innovation. Investment in local hospitals, clinics, and research laboratories will help foster new and creative drug delivery systems.
Key Controlled Release Drug Delivery System Company Insights
The controlled release drug delivery industry is highly competitive, with key players such as Johnson & Johnson, Pfizer Inc., and Merck & Co., Inc. The major companies undertake various organic and inorganic strategies, such as product introduction, collaborations, and regional expansion, to serve the unmet needs of their customers.
Key Controlled Release Drug Delivery System Companies:
The following are the leading companies in the controlled release drug delivery system market. These companies collectively hold the largest market share and dictate industry trends.
- Johnson & Johnson
- Pfizer Inc.
- Merck & Co., Inc.
- AbbVie Inc.
- Novartis AG
- Bayer AG
- AstraZeneca plc
- Corium International, Inc
- Alkermes plc
- Amneal Pharmaceuticals
Recent Developments:
-
In January 2025, Amneal Pharmaceuticals achieved FDA approval for several products: memantine/donepezil extended-release capsules for Alzheimer’s, everolimus tablets for TSC-related tumors, and tentative approval for rifaximin to treat IBS-D. The company also secured 180-day exclusivity for memantine/donepezil, broadening its accessible, complex drug range in neurology, oncology, and gastrointestinal areas within the U.S.
-
In November 2024, Alkermes plc presented 12 posters at major neuroscience conferences highlighting LYBALVI, ARISTADA, and ALKS 2680 research. A real-world study showed significant reductions in hospital admissions and emergency visits for schizophrenia and bipolar I patients after LYBALVI initiation, demonstrating its positive impact on healthcare resource utilization and treatment patterns.
-
In August 2024, Amneal Pharmaceuticals received FDA approval for CREXONT (carbidopa and levodopa) extended-release capsules for Parkinson’s disease. Combining immediate and extended-release components, CREXONT offers longer “Good On” time with fewer doses than immediate-release versions. Its U.S. launch is planned for September 2024, reinforcing Amneal’s leadership in Parkinson’s treatment innovation.
Controlled Release Drug Delivery Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 65.85 billion
Revenue forecast in 2033
USD 148.59 billion
Growth rate
CAGR of 10.6% from 2025 to 2033
Base year for estimation
2024
Historical data
2021 - 2023
Forecast period
2025 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Technology, release mechanism, application, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Switzerland; The Netherlands; Russia; Sweden; Belgium; China; India; Japan; Thailand; South Korea; Indonesia; Philippines; Vietnam; Malaysia; Singapore; Mexico; Brazil; Colombia; Argentina; Chile; South Africa; Saudi Arabia; UAE; Kuwait; Israel
Key companies profiled
Johnson & Johnson; Pfizer Inc.; Merck & Co., Inc.; AbbVie Inc.; Novartis AG; Bayer AG; AstraZeneca plc; Corium International, Inc; Alkermes plc; Amneal Pharmaceuticals
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Controlled Release Drug Delivery Market Report Segmentation
This report forecasts country-level revenue growth and analyzes the latest industry trends and opportunities in each sub-segment from 2021 to 2033. For this study, Grand View Research has segmented the global controlled release drug delivery market report based on technology, release mechanism, and region:
-
Technology Outlook (Revenue, USD Million, 2021 - 2033)
-
Wurster Technique
-
Coacervation
-
Micro Encapsulation
-
Implants
-
Transdermal
-
Targeted Delivery
-
Others (Microelectromechanical Technology, Liposomes)
-
-
Release Mechanism Outlook (Revenue, USD Million, 2021 - 2033)
-
Polymer Based Systems
-
Micro Reservoir Partition Controlled Drug Delivery Systems
-
Feedback Regulated Drug Delivery Systems
-
Activation-modulated Drug Delivery Systems
-
Chemically Activated
-
-
Activation-modulated Drug Delivery Systems Outlook (Revenue, USD Million, 2021 - 2033)
-
Osmotic Pressure Activated
-
Hydrodynamic Pressure Activated
-
Vapor Pressure Activated
-
Mechanically Activated
-
Magnetically Activated
-
-
Chemically Activated (Revenue, USD Million, 2021 - 2033)
-
pH Activated
-
Hydrolysis Activated
-
Enzyme Activated
-
-
Application Outlook (Revenue, USD Million, 2021 - 2033)
-
Metered Dose Inhalers
-
Injectable
-
Transdermal and Ocular Patches
-
Infusion Pumps
-
Oral Controlled-Drug Delivery Systems
-
Drug-Eluting Stents
-
-
Regional Outlook (Revenue, USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Netherland
-
Switzerland
-
Russia
-
Belgium
-
Sweden
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Thailand
-
South Korea
-
Indonesia
-
Philippines
-
Vietnam
-
Singapore
-
Malaysia
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
Colombia
-
Chile
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
Israel
-
-
Frequently Asked Questions About This Report
b. The global controlled release drug delivery market size was estimated at USD 59.8 billion in 2024 and is expected to reach USD 65.86 billion in 2025.
b. The global controlled release drug delivery market is expected to grow at a compound annual growth rate of 10.7% from 2025 to 2033 to reach USD 148.6 billion by 2033.
b. North America dominated the controlled release drug delivery market with a share of 42.6% in 2024. This is attributable to the rising burden of chronic diseases such as cancer, diabetes, and cardiac disorders.
b. Some key players operating in the controlled release drug delivery market include Johnson & Johnson, Pfizer Inc., Merck & Co., Inc., AbbVie Inc., Novartis AG, Bayer AG, AstraZeneca plc, Corium International, Inc, Alkermes plc, and Amneal Pharmaceuticals
b. Key factors that are driving the controlled release drug delivery market growth include the increasing preference of physicians for controlled release systems owing to benefits such as high-therapeutic efficacy, better patient compliance, and reduced treatment cost.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










