- Home
- »
- Pharmaceuticals
- »
-
Enzyme Replacement Therapy Market Size Report, 2019-2026GVR Report cover
![Enzyme Replacement Therapy Market Size, Share & Trends Report]()
Enzyme Replacement Therapy Market Size, Share & Trends Analysis Report By Enzyme (Pancreatic Enzymes, Agalsidase Beta), By Therapeutic Condition, By Route Of Administration, By End Use, And Segment Forecasts, 2019 - 2026
- Report ID: GVR-3-68038-999-9
- Number of Pages: 125
- Format: Electronic (PDF)
- Historical Range: 2015 - 2017
- Industry: Healthcare
Report Overview
The global enzyme replacement therapy market size was valued at USD 6.74 billion in 2018 and is expected to grow at a compound annual growth rate (CAGR) of7.7% from 2019 to 2026. The rising availability of enzyme replacement therapies, increasing awareness, and less stringent guidelines for rare diseases are key factors driving market potential. According to the Genetic and Rare Diseases Information Center, a rare disease is defined as a disease that affects lesser than 200,000 individuals. Strategic initiatives are being undertaken by various organizations to create awareness regarding these diseases to increase patients’ lifespan.
According to estimates from Genetic Alliance UK, a single rare disease is estimated to affect over 30,000 individuals in the UK. As a majority of chronic diseases are life-threatening, timely medical treatment is crucial to decrease mortality rates. The most commonly occurring lysosomal storage diseases include Gaucher, Fabry, Pompe, various forms of MPS, and SCID diseases. Rare diseases such as these occur in low frequencies. For instance, according to Orphanet, Gaucher’s disease is estimated to be prevalent in 1 individual in over 100,000 individuals. The low prevalence of rare or orphan diseases is one of the key factors for its limited growth in the pharmaceutical industry.
Rising awareness and an increasing number of market players venturing into orphan disease management are key factors propelling the market growth. Orphan drugs that are used to treat these diseases are anticipated to flourish during the forecast period owing to the rising number of players investing in R&D to treat these rare diseases.
The Orphan Drug Act of 1983 is a U.S. public law that has been crucial in the development of novel drugs for the treatment of Tourette syndrome myoclonus, ALS, muscular dystrophy, and Huntington's disease. Following the approval of the act, several regions such as Australia, the European Union, and Japan followed suit to facilitate enhanced treatment for orphan diseases, propelling the market growth.
Enzyme Type Insights
Based on enzyme type, the market is segmented into pegademase, velaglucerase alfa, agalsidase beta, imiglucerase, taliglucerase, laronidase, alglucosidase alfa, galsulfase, idursulfase, pancreatic enzymes, and other enzymes. The highest prevalence of Gaucher disease is one of the key factors for a large market share of enzymes such as imiglucerase and velaglucerase alfa.
Rising use of pancreatic enzymes such as amylase, lipase, and protease are used for the treatment of exocrine pancreatic insufficiency (EPI) seen commonly in cancer patients. Estimates from the American Cancer Society indicate that over 55,440 individuals were anticipated to be diagnosed with pancreatic cancer in the year 2018. Rising incidence of pancreatic cancer is thus expected to drive market growth during the forecast period.
Agalsidase beta which is used for the treatment of Fabry’s disease is anticipated to witness a CAGR of 14.1% during the forecast period. Sanofi Genzyme’s Fabrazyme is an enzyme replacement therapy used for the treatment of inherited disease. It works by replacing the enzyme responsible for low levels of GL-3 which is absent or deficient in individuals suffering from Fabry’s disease.
As the majority of these diseases are genetically acquired, early treatment is key in decreasing mortality rates. Rising awareness related to these diseases has been key in the increasing demand for enzyme replacement therapy products. Thus, enzymes are anticipated to witness lucrative growth over the forecast period.
Therapeutic Condition Insights
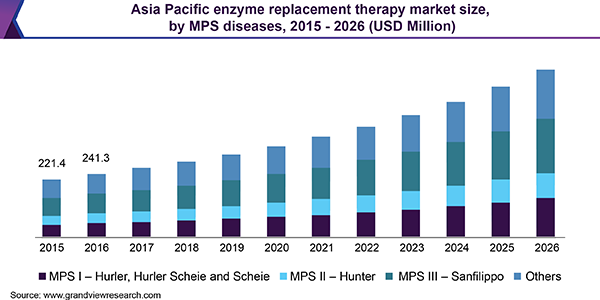
Based on therapeutic conditions, the market is divided into Gaucher’s disease, Fabry’s disease, Pompe’s disease, SCID, MPS, and others. MPS is further segmented into MPS I - Hurler, Hurler Scheie and Scheie, MPS II - Hunter, MPS III - Sanfilippo, and others. A high incidence of Gaucher’s disease is attributable to its large market share.
Gaucher disease is an inherited disease caused due to recessive mutation. According to statistics from the U.S. National Library of Medicine the disease is estimated to occur in one individual in 50,000 to 100,000 individuals of the general population, based on the region. According to data from Clinicaltrials.gov, over 60 studies are being active for Gaucher’s disease primarily funded by pharmaceutical companies.
Diseases such as Fabry, Pompe, and MPS are also seen to be gaining traction in the market owing to rising awareness brought about by organizations such as NORD (National Organization for Rare Disorders), Global Genes, GARD (Genetic and Rare Diseases Information Center) and National Institutes of Health among others.
Thus, rising awareness among pharmaceutical companies and the general public are anticipated to further propel market growth soon. Diseases such as SCID and MPS are anticipated to gain considerable market share during the forecast period owing to the increasing number of large pharmaceutical companies such as Pfizer and Shire investing heavily in the development of rare disease drugs.
Route of Administration Insights
Based on the route of administration, the enzyme replacement therapy market is divided into oral and parenteral routes. Parenteral route of administration held the largest market share in 2018 owing to its ability to facilitate rapid absorption and drug delivery. A large number of market players offer a wide range of parenteral enzyme replacement therapy; thus, this segment is anticipated to witness the highest CAGR during the forecast period.
Convenience in terms of storage, pre-measured doses, portability, and non-invasive nature of this route are some of the major factors that further accelerating oral drug adoption in the market. For instance, ZAVESCA is an oral drug developed by Actelion Pharmaceuticals Ltd which is used to treat Gaucher’s disease.
A considerable number of sustained and controlled release dosages are being preferred for the treatment of lysosomal storage diseases. These dosages tend to have a longer therapeutic effect leading to their preference over other conventional forms of drug delivery. Technologies such as microencapsulation and targeted delivery are being considered as options for enzyme treatment therapy.
End-Use Insights
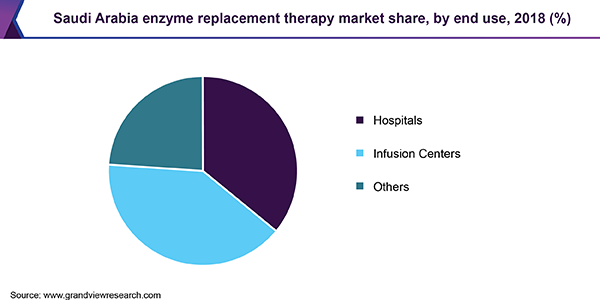
Based on end-use, the market is segmented into hospitals, infusion centers, and others. Hospitals are the primary care settings facilitating easy treatment for a wide range of diseases. Thus, these end-use settings held the largest market share owing to frequent visits by patients and their ability to cater to a varied range of lysosomal storage diseases.
Infusion centers are anticipated to gain the highest CAGR during the forecast period. Since these centers are specialized centers for IV infusions, they facilitate easy treatment for a wide range of inherited diseases. In some instances, specialized staff performs infusions at home enhancing the patient experience.
Other end-use settings that offer enzyme replacement therapy consist of specialty clinics and home care settings. These are also anticipated to gain considerable market share during the forecast period owing to effective therapies and easy treatment options. Fabry@Home is a home enzyme therapy program that involves a professional team of registered nurses and physicians. Such initiatives are typically applicable for geriatric and pediatric populations wherein home infusion proves to be an easy and effective option.
Regional Insights
North America dominated the market in 2018. Well-developed healthcare infrastructure and the presence of leading market players in North America have fueled the growth of the regional segment. Moreover, the adoption of advanced technologies and increased focus on drug development in this region is expected to drive the sector growth.
The European region accounted for the second-highest revenue after North America and is expected to grow with a CAGR of 7.1%. The healthcare system in the European region is publicly funded and some countries have adopted universal health care systems. The presence of a large number of patients suffering from MPS and SCID are factors propelling market growth in the European region.
According to statistics from the Fabry Outcome Survey prepared by Shire Outcome Surveys, Germany, Japan, and the UK exhibit the highest incidence of Fabry’s disease. Data from FDA’s Novel Drug Approvals indicate that over 39% of novel drugs approved in the year 2017 were for orphan diseases. Factors such as these are anticipated to further propel regional market growth.
Asia Pacific region consists of developed as well as developing countries such as China, India, and other Asia Pacific countries. Most of the countries in this region are economically developing and the disposable income is gradually increasing. Many multinational companies are heavily investing in this region. Besides, countries such as Japan are technologically advanced in terms of healthcare and medical research, which aids in the market growth of this region.
Key Companies & Market Share Insights
The market constitutes a large number of global as well as local players. The majority of these market players have a well-established portfolio of lysosomal storage diseases facilitating easy and effective treatment.
Some of the leading market players, which include Shire Plc, Sanofi S.A., AbbVie, and Allergan plc, have a strong geographical presence in terms of a distribution network and operating facilities. Key players are mainly focused on developing and marketing drugs solely focused on rare and genetic diseases. For instance, the Shire, plc has over 40 products that are sold globally solely centered around rare and orphan diseases. The company is also focused on creating significant awareness related to these diseases and hence constantly undertakes various campaigns to facilitate cognizance. Rare Count is one such imitative launched by the company in February 2017. This initiative was launched to create awareness of the orphan disease among the general population.
Also, mergers, acquisitions, and strategic agreements are some of the initiatives being undertaken by key players, which are expected to propel the enzyme replacement therapy market. The majority of the players are focusing on the development of new products. Various companies are also engaged in the licensing and distribution of drugs to aid patients more effectively. Some of the prominent players in the enzyme replacement therapy market include:
-
Shire Plc; Sanofi S.A.
-
Biomarin Pharmaceutical Inc.
-
AbbVie
-
Alexion Pharmaceuticals Inc.
-
Allergan plc
-
Horizon Pharma Public Limited Company
-
Actelion (Janssen)
-
Recordati Rare Diseases
-
Protalix Biotherapeutics
-
Amicus Therapeutics, Inc.
Enzyme Replacement Therapy Market Report Scope
Report Attribute
Details
The market size value in 2020
USD 7.70 billion
The revenue forecast in 2026
USD 12.3 billion
Growth Rate
CAGR of 7.7% from 2019 to 2026
The base year for estimation
2018
Historical data
2015 - 2017
Forecast period
2019 – 2026
Quantitative units
Revenue in USD million and CAGR from 2019 to 2026
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
The enzyme, therapeutic condition, route of administration, end-user, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Russia; Japan; India; China; South Korea; Australia; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE
Key companies profiled
Shire Plc; Sanofi S.A.; Biomarin Pharmaceutical Inc.; AbbVie; Alexion Pharmaceuticals Inc.; Allergan plc; Horizon Pharma Public Limited Company; Actelion (Janssen); Recordati Rare Diseases; Protalix Biotherapeutics; Amicus Therapeutics, Inc.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Segments Covered in the reportThis report forecasts revenue growth at global, regional & country levels and provides an analysis of the industry trends in each of the sub-segments from 2015 to 2026. For this study, Grand View Research has segmented the global enzyme replacement therapy market report based on the enzyme, therapeutic condition, route of administration, end-user, and region:
-
Enzyme Outlook (Revenue, USD Million; 2015 - 2026)
-
Imiglucerase
-
Agalsidase Beta
-
Taliglucerase
-
Velaglucerase Alfa
-
Laronidase
-
Alglucosidase Alfa
-
Galsulfase
-
Idursulfase
-
Pancreatic Enzymes
-
Pegademase
-
Others
-
-
Therapeutic Condition Outlook (Revenue, USD Million; 2015 - 2026)
-
Gaucher Disease
-
Fabry Disease
-
Pompe Disease
-
SCID
-
MPS
-
MPS I - Hurler, Hurler Scheie and Scheie
-
MPS II - Hunter
-
MPS III - Sanfilippo
-
Others
-
-
Others
-
-
Route of Administration Outlook (Revenue, USD Million; 2015 - 2026)
-
Oral
-
Parenteral
-
-
End-Use Outlook (Revenue, USD Million; 2015 - 2026)
-
Hospitals
-
Infusion Centers
-
Others
-
-
Regional Outlook (Revenue, USD Million; 2015 - 2026)
-
North America
-
The U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Russia
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
MEA
-
South Africa
-
Saudi Arabia
-
UAE
-
-
Frequently Asked Questions About This Report
b. The global enzyme replacement therapy market size was estimated at USD 7.20 billion in 2019 and is expected to reach USD 7.70 billion in 2020.
b. The global enzyme replacement therapy market is expected to grow at a compound annual growth rate of 7.7% from 2018 to 2026 to reach USD 12.3 billion by 2026.
b. Gaucher's disease dominated the enzyme replacement therapy market with a share of 32.09% in 2019. This is attributable to the high incidence of Gaucher’s disease globally.
b. Some key players operating in the enzyme replacement therapy market Shire Plc, Sanofi S.A., Biomarin Pharmaceutical Inc., AbbVie, Alexion Pharmaceuticals Inc., Allergan plc, Horizon Pharma Public Limited Company, Actelion (Janssen), Recordati Rare Diseases, Protalix Biotherapeutics, and Amicus Therapeutics, Inc.
b. Key factors driving the market growth include rising availability of enzyme replacement therapies, increasing awareness, and less stringent guidelines for rare diseases.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





