- Home
- »
- Medical Devices
- »
-
Heart Pump Device Market Size, Industry Report, 2030GVR Report cover
![Heart Pump Device Market Size, Share & Trends Report]()
Heart Pump Device Market (2025 - 2030) Size, Share & Trends Analysis Report By Type, By Product (Ventricular Assist Devices, Intra-Aortic Balloon Pumps, Extracorporeal Membrane Oxygenation), By End-use, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-071-5
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Heart Pump Device Market Summary
The global heart pump device market size was estimated at USD 2.9 billion in 2024 and is projected to reach USD 6.39 billion by 2030, growing at a CAGR of 13.6% from 2025 to 2030. The increasing prevalence of heart diseases, such as coronary heart disease and cardiovascular disease, the growing demand for technologically advanced products, and growing approval from regulatory bodies are propelling the industry's growth.
Key Market Trends & Insights
- North American dominated the heart pump device market with a revenue share of 52.59% in 2024.
- The U.S. heart pump devices market dominated with a revenue share of 88.5% in 2024.
- By type, implanted heart pump devices segment accounted for the largest share of 69.1% in 2024.
- By product, ventricular assist devices (VADs) segment held the largest market share of 68.5 % in 2024.
- By end use, the hospital segment dominated the market in 2024.
Market Size & Forecast
- 2024 Market Size: USD 2.9 Billion
- 2030 Projected Market Size: USD 6.39 Billion
- CAGR (2025-2030): 13.6%
- North America: Largest market in 2024
According to the CDC, approximately 12.1 million Americans will be suffering from atrial fibrillation by 2030.In 2022, there were 315 million cases of coronary artery disease worldwide. The increase in heart disease cases can be attributed to lifestyle changes and an aging population.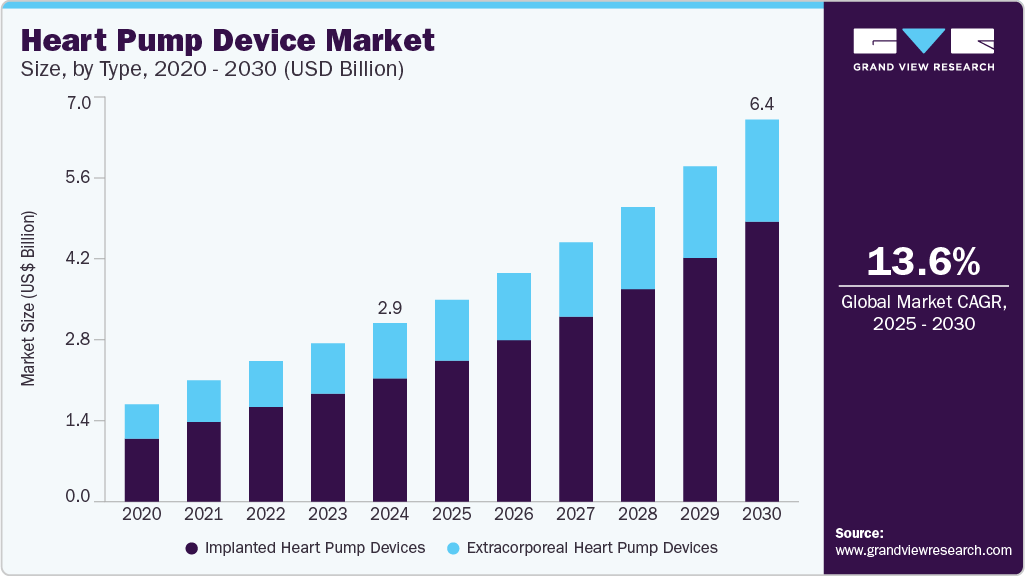
The increasing regulatory approval of heart pump devices is expected to boost market growth. In December 2024, J & J MedTech announced that the USFDA expanded the indications of the Impella 5.5 SmartAssist and Impella CP SmartAssist heat pumps for use in pediatric patients having symptomatic acute decompensated heart failure and cardiogenic shock, and additionally, they received premarket approval for the same.
Collaborative efforts allow the merging of knowledge and technical know-how from various participants in the industry. By combining insights from different organizations, researchers gain a more comprehensive understanding of the complex challenges associated with heart pump devices. This shared knowledge leads to more effective solutions, improved device designs, and enhanced patient outcomes.
For instance, in January 2024, Ultromics, a health technology enterprise centered on patient outcomes and propelled by AI, originating from the University of Oxford, UK, forged a collaborative agreement with Pfizer. The objective was to bolster the validation process and secure FDA clearance for Ultromics' AI-driven technology to detect cardiac amyloidosis. Within this alliance, Ultromics will undertake research to achieve FDA clearance for its EchoGo Amyloidosis algorithm, a medical device with Breakthrough Device Designation specifically designed to identify cardiac amyloidosis. The algorithm uses deep learning techniques to analyze routine echocardiograms, revealing cardiac amyloidosis cases that often evade detection during standard assessments.
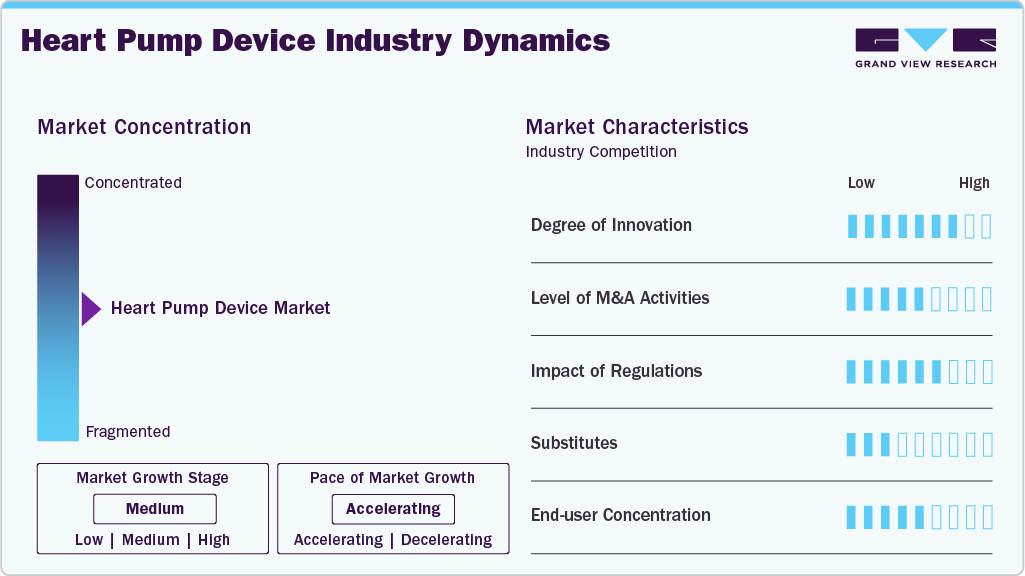
Market Concentration & Characteristics
The heart pump device market is moderately consolidated, with leading players introducing novel products. The market growth stage is high, and the pace of market growth is accelerating. In April 2025, Medtronic received approval from the US FDA for the world's smallest defibrillator lead. This device is designed to stimulate the right ventricle in adult and adolescent pediatric patients aged 12 and older, including those with smaller anatomies, marking a significant advancement in personalized cardiac care.
In July 2024, Magenta Medical Ltd. raised USD 105 million for clinical trials for the Elevate system, the world’s smallest heart pump.
The market is witnessing continuous research and development aimed at advancing cardiac care. In November 2022, LivaNova received 510(k) clearance from the USFDA for the LifeSPARC ECMO machine, allowing it to be used beyond 6 hours for patients with acute cardiopulmonary failure.
In January 2025, the BrioVAD heart pump device, developed by BrioHealth Solutions, Inc., was implanted in patients during a groundbreaking clinical trial. This trial represents a significant opportunity to evaluate cutting-edge technology as we seek to revolutionize treatment options for advanced heart failure.
A moderate level of M&A activities characterizes the heart pump device market. The key reason for the frequent acquisitions in this market is the technological strength of small players with their robust product pipelines. It allows the key players to improve their market share, expand their product portfolio, and provide opportunities to be the first entrant into a niche market segment. For instance, in December 2023, Johnson & Johnson Service Inc. acquired Abiomed, Inc. with the aim of expanding its cardiovascular portfolio. This acquisition is expected to have a positive impact on market growth.
The stringent regulatory framework for the approval and commercialization of heart pump devices impedes market growth. Furthermore, compliance with regulatory standards further results in product recall, which reduces patient and healthcare participant confidence. For instance, In April 2025, the US FDA announced a crucial recall of Abbott’s mobile power units (MPUs) used in HeartMate 3 LVAD and HeartMate II LVAD, prioritizing patient safety and ensuring exceptional performance standards are upheld.
Key companies in the heart pump devices market are also focusing on market expansion. For instance, in October 2023, CorWave SA opened a new production facility to support its commercial and clinical trial phase activities on the banks of the Seine in Clichy, next to Paris. Additionally, in March 2025, Supira Medical successfully raised USD 120 million to advance the clinical development of its innovative temporary heart pump. This groundbreaking device is specifically designed for patients facing high-risk cardiac procedures.
Type Insights
Implanted Heart Pump Devices accounted for the largest share of 69.1% in 2024. These devices assume the heart's pumping function by continuously circulating blood from the ventricles throughout the body. The rising prevalence of heart failure and technological advancements in implanted heart pump devices drives market growth. Improved durability, smaller sizes, and enhanced control systems are making these devices more accessible & effective for a wider range of patients. In April 2025, engineers at Northwestern University created the world’s smallest pacemaker, which is small enough to fit inside a syringe's tip and can be injected into the body without surgery. While it is compatible with hearts of all sizes, it is especially designed for the delicate and small hearts of newborns suffering from congenital heart defects.
The extracorporeal heart pump devices segment is also expected to grow significantly over the forecast period. Extracorporeal heart pump products are external devices that temporarily support the heart by pumping blood outside the body and returning it to the circulatory system. They effectively treat acute HF and are used as a bridge to transplantation. The rising prevalence of heart disease and the shortage of donor hearts have driven the demand for extracorporeal products.
Product Insights
Ventricular Assist Devices (VADs) held the largest market share of 68.5 % in 2024. VADs are mechanical devices that assist with pumping blood from the heart's ventricles to the rest of the body. They are typically used as a temporary solution while awaiting a heart transplant but are also increasingly being used as a long-term treatment option for HF patients who are not candidates for transplantation.
Market players' introduction of new and advanced VADs is a key factor driving the segment's growth. These products are designed to be more effective, user-friendly, and affordable, which is expected to increase the demand for VADs even further. For instance, in August 2022, Abbott Laboratories disclosed data revealing that patients with advanced heart failure who received its HeartMate 3 LVAD had higher survival rates after five years than those who received the older HeartMate II LVAD. The HeartMate 3 demonstrated a 58% survival rate over the five years, compared to the HeartMate II's 44% survival rate. This is mainly due to decreased deaths related to stroke, clotting, and bleeding associated with the newer device.
The Extracorporeal Membrane Oxygenation (ECMO) segment is expected to register the fastest CAGR of 11.3 % during the forecast period. The extracorporeal membrane oxygenation device has been growing due to advancements in technology and its ability to offer a viable solution for patients with end-stage HF awaiting a heart transplant. In September 2024, Medtronic launched the VitalFlow ECMO machine, simplifying therapy and enhancing usability.
End Use Insights
The hospital segment dominated the market in 2024. This growth can be attributed to the increasing number of surgical procedures being performed globally due to the rising incidence of cardiovascular diseases and the availability of skilled professionals and novel heart pump products. According to an NIH study in 2023, high-income countries have an average of 123.2 cardiac surgeries per 100,000 population yearly. In December 2024, KMC Hospital, in Mangaluru, partnered with Dr. Madhan's ECMO Health Care Pvt. Ltd. to introduce the ECMO services. This initiative represents a significant advancement, as it is the first of its kind in coastal Karnataka and Northern Kerala.
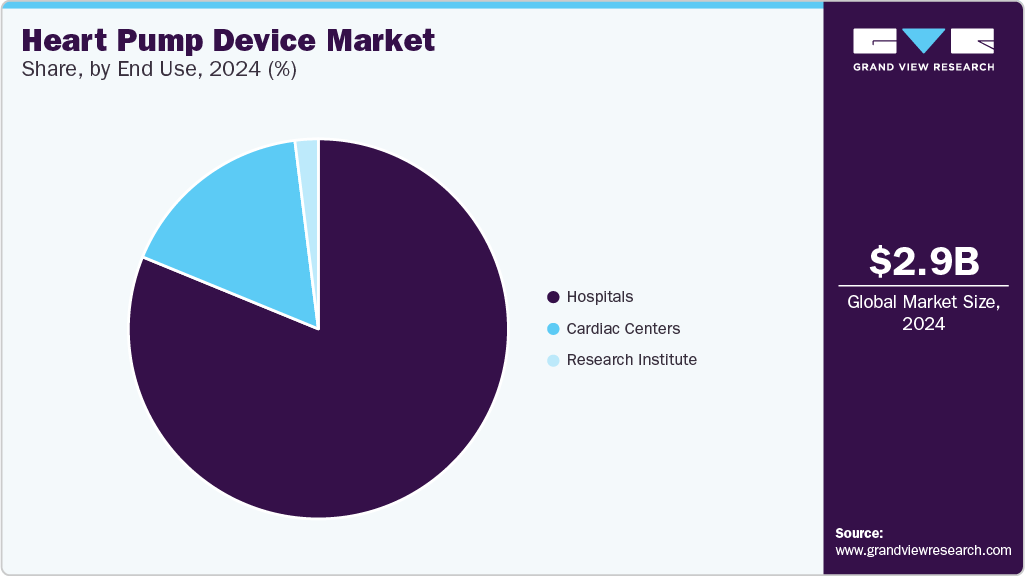
The cardiac center segment is projected to grow at the fastest CAGR of 14.7 % over the forecast period, owing to the increasing preference of individuals to undergo surgical interventions in cardiac centers. Leading cardiac centers, such as the Cleveland Clinic in the U.S., St. Thomas' Hospital in the UK, and the German Heart Center Munich in Germany, exemplify the demand for heart pump devices. These centers are renowned for their expertise in treating complex cardiac conditions and are often early adopters of cutting-edge technologies, driving the adoption of heart pump devices and shaping the market landscape.
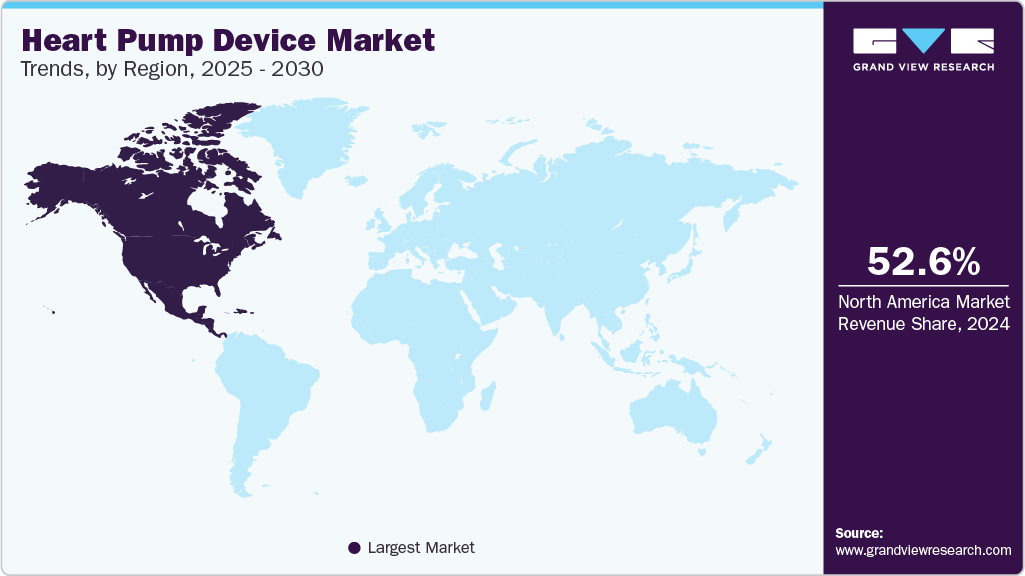
Regional Insights
North American dominated the heart pump device market with a revenue share of 52.59% in 2024. The rising incidence of cardiovascular disease, advanced healthcare infrastructure, and the presence of key players. In addition, the increasing number of patients undergoing surgeries and the rising awareness about minimally invasive surgeries are expected to propel market growth in North America.
U.S. Heart Pump Device Market Trends
The U.S. heart pump devices market dominated with a revenue share of 88.5% in 2024. In the U.S., advanced healthcare infrastructure, the growing patient pool, and the increasing number of new product launches are some of the major factors expected to propel revenue share. In December 2024, BiVacor completed the initial phase of its FDA-approved early feasibility study (EFS) for the Total Artificial Heart (TAH), marking a significant advancement in cardiac innovation.
Europe Heart Pump Device Market Trends
The Heart Pump devices market in Europe held the second-largest revenue market share in 2024. According to the WHO report, in 2024, cardiovascular diseases (CVDs) are the leading cause of disability and premature death in the European Region, accounting for over 42.5% of all deaths annually, which is equal to 10,000 deaths each day. This number is expected to rise in the coming years, indicating a growing need for advanced treatment options such as heart pumps . In May 2024, MOBYBOX, the world’s first integrated portable pneumatic ECMO machine, commercially deployed its first batch in Europe.
The heart pump device market in the UK held the second-largest market share in 2024. The prevalence of cardiovascular diseases, such as coronary artery disease, ischemic heart disease, and other heart-related disorders, is expected to increase the demand for heart pump devices.
France heart pump device market is anticipated to witness significant CAGR during the forecast period. Growth in France is likely driven by rising incidence of cardiovascular diseases, growing number of hospitals & clinics, and lifestyle changes in the population.
Asia Pacific Heart Pump Device Market Trends
Asia Pacific dominated the heart pump device market with a revenue share of 52.59% in 2024.The Asia Pacific heart pump device market is anticipated to grow at a CAGR of 14.1 % during the forecast period. Increasing health awareness, a developing private hospital sector, growing government support and spending, the rising prevalence of cardiovascular diseases, and the growing availability of insurance policies will likely contribute to market growth over the coming decade.
Heart pump device market in China held the largest revenue market share in 2024. The Annual Report on Cardiovascular Health and Diseases in China (2021) estimated that approximately 8.9 million patients in China suffer from heart failure. In addition, in March 2023, Yongrenxin Medical Instrument Co. Ltd. secured nearly USD 100 million in a series A funding to develop a platform for treating heart failures.
Japan heart pump device market held the second largest market share in the Asia Pacific region. Heart disease is the second-leading cause of mortality in Japan, with CHD accounting for about half of heart disease-related deaths. In October 2023, the Icahn School of Medicine at Mount Sinai announced a collaboration with the Chiba Institute of Technology (CIT) to use Artificial Intelligence (AI) for cardiovascular disease research.
Heart pump device market in India is expected to grow at the significant CAGR during the forecast period. Hospital-based studies conducted in Trivandrum and the All-India Institute of Medical Sciences (AIIMS) indicate that rheumatic heart disease (RHD) and coronary artery disease (CAD) are significant contributors to heart failure cases in India. Heart failure stands out as the most common reason for cardiac-related hospitalizations, affecting approximately 1% of the general population annually, which translates to an estimated 8 to 10 million patients.
Central & South America Heart Pump Device Market Trends
The heart pump device market in Latin America is driven by several factors. One key driver is the increasing prevalence of cardiovascular diseases in the region, particularly among the aging population. The rise in lifestyle-related risk factors such as obesity and diabetes also contributes to the growing demand for heart pump devices.
The heart pump device market in Mexico is expected to grow due to various factors, such as the high incidence of chronic heart diseases, a growing population, and the increasing prevalence of cardiovascular diseases. There is a rising demand for advanced cardiac therapies. The market is witnessing a shift toward more innovative and efficient devices, driven by advancements in technology and increasing awareness among healthcare providers and patients.
Middle East & Africa Heart Pump Device Market Trends
MEA heart pump devices market is expected to grow at a lucrative rate. The region faces a significant burden of cardiovascular diseases, including heart failure, which has led to an increased demand for advanced cardiac therapies. With a rising aging population and an increase in lifestyle-related risk factors, such as hypertension & diabetes, the need for innovative solutions in cardiac care is becoming more pronounced.
The heart pump device market in South Africa held the largest revenue market share in 2024. Another trend driving the market is the growing prevalence of cardiovascular diseases in South Africa, particularly among the aging population. This has created a higher demand for advanced cardiac therapies, including heart pump devices and artificial hearts, to improve patient care and outcomes.
Key Heart Pump Device Company Insights
Leading companies in the heart pump devices market are enhancing their offerings and incorporating new technologies to expand their customer reach, secure a greater market share, and diversify their application range.
Key Heart Pump Device Companies:
The following are the leading companies in the heart pump device market. These companies collectively hold the largest market share and dictate industry trends.
- Abbott
- ABIOMED (Johnson & Johnson Services, Inc.)
- Getinge
- LivaNova PLC
- Berlin Heart
- Picard Medical, Inc. (SynCardia Systems, LLC)
- Jarvik Heart, Inc.
- BiVACOR Inc.
- Leviticus Cardio
- Teleflex Incorporated.
Recent Developments
-
In March 2025, The ACC (American College of Cardiology) and AHA (American Heart Association) updated their ACS (Acute coronary syndrome) treatment guidelines, upgrading Impella use in STEMI-related cardiogenic shock from Class 2b to 2a, citing improved survival benefits.
-
In December 2024, Daya General Hospital (Kerala, India) inaugurated the ECMO service to improve the outcomes of critically ill patients.
-
In November 2024, Abbott announced the launch of the AVEIR VR single-chamber ventricular leadless pacemaker in India, designed specifically for patients experiencing slow heart rhythms.
-
In November 2023, BiVACOR Inc. received USD 13 million as a grant from the Australian Government's Medical Research Future (MRFF) under the Artificial Heart Frontiers Program (AHFP) to support clinical trials for total artificial heart devices.
-
In April 2023, Abbott obtained two new authorizations from the U.S. FDA for its leading life support system, CentriMag Blood Pump. With this clearance, the CentriMag Blood Pump can be used longer-term in adults during ECMO procedures.
Heart Pump Device Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 3.38 billion
Revenue forecast in 2030
USD 6.39 billion
Growth Rate
CAGR of 13.6% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, product, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S., Canada, Mexico, Germany, UK, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, India, Australia, Thailand, South Korea, Brazil, Argentina, UAE, Saudi Arabia, Kuwait, South Africa
Key companies profiled
Abbott; ABIOMED (Johnson & Johnson Services Inc; Getinge ;LivaNova PLC; Berlin Heart; Picard Medical Inc. (SynCardia Systems LLC); Jarvik Heart; BiVACOR Inc.; Leviticus Cardio; Teleflex Incorporated.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Heart Pump Device Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global heart pump device market report based on type, product, end use, and region.
-
Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
Implanted Heart Pump Devices
-
Extracorporeal Heart Pump Devices
-
-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Ventricular Assist Devices (VADs)
-
Left Ventricular Assist Devices (LVADs)
-
Right Ventricular Assist Devices (RVADs)
-
BiVAD Ventricular Assist Devices (BiVADs)
-
Percutaneous Ventricular Assist Devices (PVADs)
-
-
Intra-Aortic Balloon Pumps (IABPs)
-
Extracorporeal Membrane Oxygenation (ECMO)
-
-
End Use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hospitals
-
Cardiac Centers
-
Research Institute
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










