- Home
- »
- Medical Devices
- »
-
Intravascular Catheters Market Size Report, 2019-2025GVR Report cover
![Intravascular Catheters Market Report]()
Intravascular Catheters Market (2019 - 2025) Analysis Report By Product (Short, Integrated/Closed PIVCs), By Application, By End Use (Hospitals, Clinics, ASC, Homecare), By Region, And Segment Forecasts
- Report ID: GVR-2-68038-744-5
- Number of Report Pages: 85
- Format: PDF
- Historical Range: 2014 - 2017
- Forecast Period: 2019 - 2025
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Report Overview
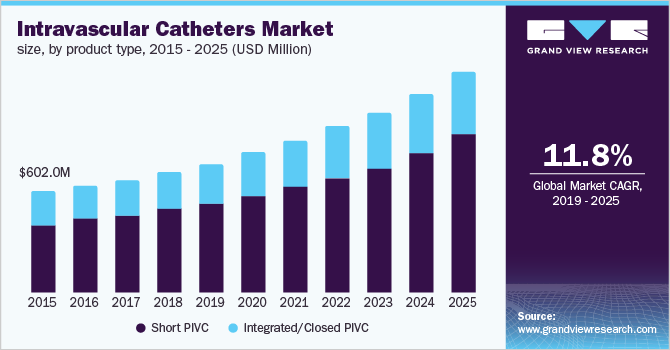
The global intravascular catheters market size to be valued at USD 8.8 billion by 2025 and is expected to grow at a compound annual growth rate (CAGR) of 11.8% during the forecast period. Key factors driving the market include increasing incidence of cardiovascular disorders, neurological disorders, and urinary tract infections, among others.
The market is also fueled by rising hospitalization rate owing to high prevalence of chronic diseases, increasing number of ambulatory care centers and catheterization labs, and growing use of catheters for minimally invasive diagnostic and interventional procedures. Growing prevalence of kidney failure, diseases associated with urinary bladder, and other urological conditions are major factors driving the market. A large global geriatric population translates to high rate of incontinence problems. This has resulted in rising demand for high quality, sterile catheters, thereby contributing to market growth.
Intravascular catheters have become an indispensable component in everyday medical practices and are of utmost importance in intensive care units (ICUs). While their role in providing vascular access is crucial, they can also put patients at risk for various infectious complications, including catheter-related bloodstream infections (CRBSI), local site infections, endocarditis, septic thrombophlebitis, and other metastatic infections. This risk of morbidity and mortality presented by intravascular catheters has been a cause for concern within the healthcare industry, posing a challenge to market growth. However, technological advancements are anticipated to eliminate associated infections, thereby increasing demand for the same over the forecast period.
Price competition, inadequate quality assurance, and restricted reimbursement scenario in several countries are some of the other hurdles the market has had to face. To tackle this, various regulations and policies have been introduced by governments of respective countries to provide medical cover to millions of new patients. Programs such as the Child Health Insurance Program (CHIP) in U.S., which provides low-cost health coverage to children, and in some cases/states pregnant women, are anticipated to boost the market over the forecast period.
Intravascular Catheters Market Trends
The use of intravascular catheters for measuring pH, blood pressure, and other health parameters in youngsters has been critical to the global industry growth. Intravascular catheters are also used to treat diseases of the urinary bladder and neurology. As a result, the global intravascular catheter market is expected to expand quickly during the forecast period.
With the increased prevalence of urological and cardiovascular illnesses, the intravascular catheter market is expected to grow significantly. Additionally, the quick technological advancement of intravascular catheters and the expanding number of catheterization labs drive the industry growth.
The risk of bloodstream infections associated with intravascular catheter usage, and a scarcity of skilled healthcare workers, are two major factors restricting the growth of the global market.
The governments have enacted numerous laws and regulations to provide medical care to millions of people. Programs like the Child Health Insurance Program in the United States (CHIP), which provides low-cost coverage for children and, in some cases, pregnant women, are expected to contribute to the industry growth over the forecast period. The infectious diseases segment would grow the most with the increased prevalence of infectious diseases such as HIV, tuberculosis, and HPV. As these diseases become more widespread, hospital admissions and healthcare providers will improve. As a result, the intravascular catheter market gets significant opportunities over the forecast period.
Product Type Insights
Based on product, the market is classified into short peripheral intravenous catheters (PIVC) and integrated PIVC. In 2018, short PVIC dominated the market with a revenue of USD 2.8 billion and is anticipated to grow at the fastest pace over the forecast period. This can be attributed to the added advantage of fewer infections associated with these PIVCs. In addition, factors such as increasing incidence of cardiovascular, neurological, oncological, and gastroenterological diseases are anticipated to drive demand for PIVC in surgical intervention and diagnostics.
Application Insights
On the basis of application, the intravascular catheters market is classified into oncology, gastroenterology, renal disease, infectious diseases, and others. The others segment consists of surgical interventions performed owing to trauma, and other diseases such cardiovascular and urological diseases. In 2018, this segment dominated the market with a revenue of USD 1.7 billion and is expected to grow at a lucrative rate over the forecast period.
The infectious diseases segment is expected to register the highest growth, exhibiting a CAGR of 15.0% over the forecast period owing to increasing incidence of infectious diseases such as HIV, tuberculosis, and HPV. According to U.K.-based HIV and AIDS charity Avert, about 36.9 million people were found to be living with HIV across the globe. Furthermore, according to the World Health Organization (WHO) in 2017, about 10 million people were diagnosed with tuberculosis worldwide, resulting in an estimated 1.6 million deaths. Increasing incidence of these diseases is expected to raise the admission rate in hospitals and other healthcare providers, thereby spurring demand for intravascular catheters.
End-use Insights
On the basis of end use, the market is segmented into hospitals, clinics, Ambulatory Surgical Centers (ASC), homecare, and others. In 2018, the hospitals segment dominated the market with a revenue of USD 2.5 billion and is anticipated to grow at a lucrative rate over the forecast period. This is owing to high number of surgical procedures being performed for the treatment of cardiovascular, gastroenterology, renal and other infectious diseases.
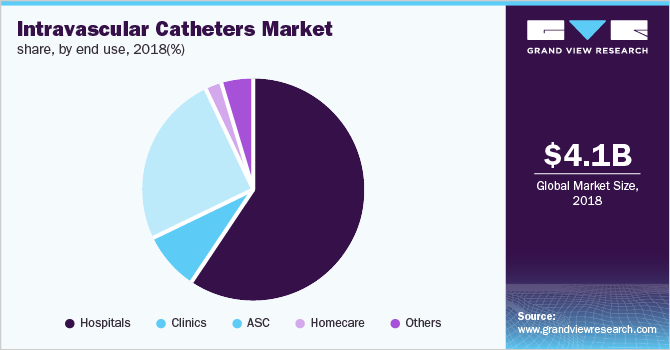
The ambulatory surgical centers (ASC) segment is anticipated to grow at the fastest pace, registering a CAGR of 13.5% over the forecast period. ASCs are independent healthcare facilities entirely focused on providing outpatient surgical care. They incur comparatively less surgery costs when compared to those in hospitals. Moreover, these ASCs are comparatively cleaner and safer in comparison with hospitals and hence are associated with fewer infections. These factors, coupled with increasing incidence of cardiovascular, neurological, oncological, and gastroenterological diseases, are anticipated to drive demand for intravascular catheters in ASCs.
Regional Insights
On the basis of region, the market is segmented into North America, Europe, Asia Pacific, Latin America, and Middle East and Africa (MEA). Asia Pacific dominated the market in 2018, with a value of USD 1.2 billion, and is expected to grow at a lucrative rate over the forecast period. Some of the key reasons attributed to Asia Pacific’s large market share are growing prevalence of oncological disorders, infectious and renal diseases, and urological problems and rising inpatient and emergency room procedures. Moreover, a large population base is anticipated to drive the market in Asia Pacific over the forecast period.
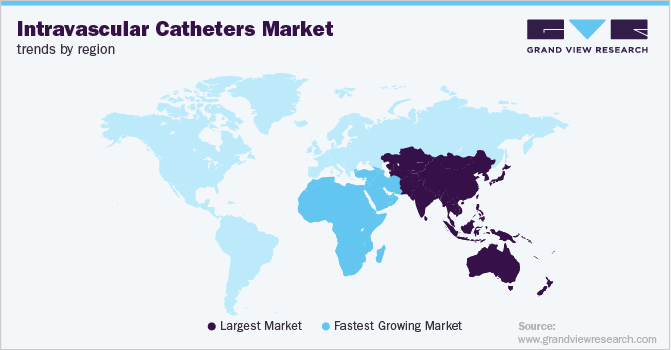
MEA is expected to grow at the fastest rate, with a CAGR of 13.3 % over the forecast period.Key factors driving the regional market include increasing prevalence of cardiovascular diseases and other anomalies in low- and middle-income countries. Moreover, constantly improving healthcare infrastructure, increasing healthcare expenditure levels, and presence of high unmet medical needs are expected to serve as high-impact rendering drivers to the MEA market.
Key Companies & Market Share Insights
The market is highly competitive and comprises a network of players engaged in activities such as research and product development, medical device manufacturing, raw material supply, sales and distribution, and even post-sales services. Technological variation is one of the key criteria that most players compete on. Some of the key players operating in this market are Cook Medical; Smiths Medical; Edwards Life Sciences Corporation; Medtronic Inc.; Johnson & Johnson; McKesson Medical Surgical Inc.; and Terumo Corporation.
Recent Developments
-
In May 2022, Cook Medical launched the MINC+ Benchtop Incubator for IVF facilities in the United States and Canada. The MINC+ is the next generation of the Mini Incubator. The DishTrace technology, which combines capability through the incubator screen and computer system software to provide a wide range of dish-data management features, is also included with the MINC+ incubator.
-
In December 2021, Edwards Lifesciences stated that the FDA had authorized the usage of the Edwards SAPIEN 3 transcatheter valve with the Alterra adaptive prestent for people with acute pulmonary regurgitation.
Intravascular Catheters Market Report Scope
Report Attribute
Details
Market size value in 2020
USD 4.5 billion
Revenue forecast in 2025
USD 8.8 billion
Growth Rate
CAGR of 11.8% from 2019 to 2025
Base year for estimation
2018
Historical data
2014 - 2017
Forecast period
2019 - 2025
Quantitative units
Revenue in USD million and CAGR from 2019 to 2025
Report coverage
Revenue forecast, company share, competitive landscape, growth factors and trends
Segments covered
Product, application, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; U.K.; Spain; France; Italy; Russia; Japan; China; India; South Korea; Singapore; Australia; Mexico; Brazil; Argentina; South Africa; Saudi Arabia; UAE
Key companies profiled
Cook Medical; Smiths Medical; Edwards Life Sciences Corporation; Medtronic Inc.; Johnson & Johnson; McKesson Medical Surgical Inc.; Terumo Corporation
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
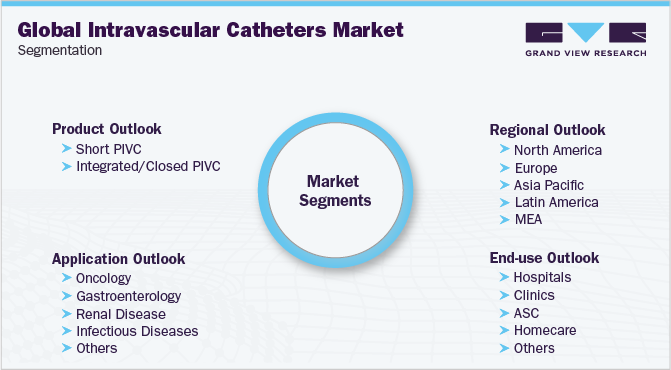
Global Intravascular Catheters Market SegmentationThis report forecasts revenue growth at global, regional, and country levels and provides an analysis on latest industry trends in each of the sub-segments from 2014 to 2025. For the purpose of this study, Grand View Research has segmented the global intravascular catheters market report on the basis of product, application, end use, and region.
-
Product Outlook (Revenue, USD Million, 2014 - 2025)
-
Short PIVC
-
Integrated/Closed PIVC
-
-
Application Outlook (Revenue, USD Million, 2014 - 2025)
-
Oncology
-
Gastroenterology
-
Renal Disease
-
Infectious Diseases
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2014 - 2025)
-
Hospitals
-
Clinics
-
ASC
-
Homecare
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2014 - 2025)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
U.K.
-
Spain
-
France
-
Italy
-
Russia
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Singapore
-
Australia
-
-
Latin America
-
Mexico
-
Brazil
-
Argentina
-
-
MEA
-
Saudi Arabia
-
South Africa
-
UAE
-
-
Frequently Asked Questions About This Report
b. The global intravascular catheters market size was estimated at USD 4.1 billion in 2019 and is expected to reach USD 4.5 billion in 2020.
b. The global intravascular catheters market is expected to grow at a compound annual growth rate of 11.8% from 2019 to 2025 to reach USD 8.8 billion by 2025.
b. The Asia Pacific dominated the intravascular catheters market with a share of 28.3% in 2019. This is attributable to the growing prevalence of oncological disorders, infectious and renal diseases, and urological problems and rising inpatient and emergency room procedures.
b. Some key players operating in the intravascular catheters market include Cook Medical; Smiths Medical; Edwards Life Sciences Corporation; Medtronic Inc.; Johnson & Johnson; McKesson Medical Surgical Inc.; and Terumo Corporation.
b. Key factors that are driving the market growth include the increasing prevalence of cardiovascular diseases, and end-stage renal disease.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










