- Home
- »
- Medical Devices
- »
-
IV Tubing Sets And Accessories Market Size Report, 2030GVR Report cover
![IV Tubing Sets And Accessories Market Size, Share & Trends Report]()
IV Tubing Sets And Accessories Market (2025 - 2030) Size, Share & Trends Analysis Report By Product (Primary, Secondary, Extension, Accessories), By Application, By End-use, By Region, And Segment Forecasts
- Report ID: GVR-4-68038-191-7
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
IV Tubing Sets And Accessories Market Summary
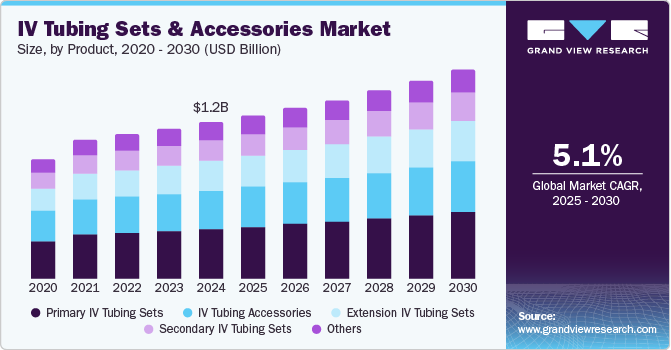
The global IV tubing sets and accessories market size was estimated at USD 1.24 billion in 2024 and is projected to reach USD 1.65 billion by 2030, growing at a CAGR of 5.1% from 2025 to 2030. Market growth worldwide is driven by the escalating prevalence of chronic diseases such as diabetes, cancer, and cardiovascular conditions.
Key Market Trends & Insights
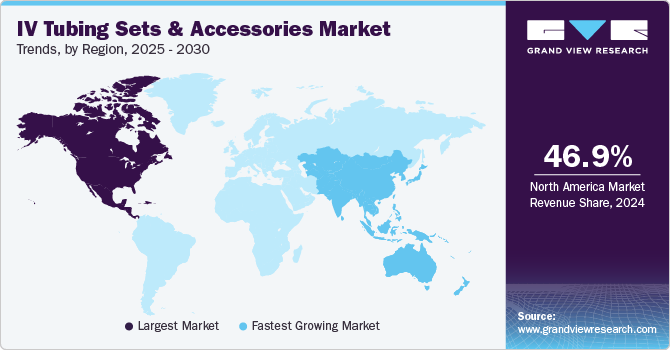
- North America dominated the global IV tubing sets and accessories market with the largest revenue share of 46.9% in 2024.
- By product, the primary IV tubing sets segment led the market with the largest revenue share of 32.2% in 2024.
- By product, the peripheral intravenous catheter insertion segment led the market with the largest revenue share of 48.5% in 2024,
- By end use, the ambulatory surgical centers (ASCs) segment is projected to grow at the fastest CAGR of 5.8% over the forecast period.
Market Size & Forecast
- 2024 Market Size: USD 1.24 Billion
- 2030 Projected Market Size: USD 1.65 Billion
- CAGR (2025-2030): 5.1%
- Largest Market: North America
With the NCBI’s reports of around 129 million people in the U.S. requiring long-term intravenous therapies, there is increasing demand for reliable and efficient IV solutions for effective patient management.
Advancements in healthcare infrastructure and the rising trend toward home-based care are significantly influencing the IV tubing sets market. The COVID-19 pandemic has accelerated the transition to home healthcare services, providing patients with a cost-effective alternative to prolonged hospital stays for IV therapy. Companies such as B. Braun Melsungen AG have effectively leveraged this trend by launching portable and user-friendly IV devices tailored for at-home use. As the elderly population increases, the preference for home healthcare further enhances the demand for these innovative products.
Furthermore, the introduction of features such as needleless connectors, anti-kink tubing, and smart IV pumps enhances the safety and efficiency of IV therapy. For instance, in July 2023, BD announced FDA 510(k) clearance for the updated BD Alaris Infusion System, optimizing infusion therapy to improve health system efficiency. Such systems ensure accurate medication delivery, enhance patient outcomes, and encourage healthcare providers to upgrade their IV administration capabilities, increasing operational efficiency.
Moreover, robust regulatory frameworks in regions such as North America ensure the highest quality standards for medical devices, instilling confidence among healthcare providers and patients alike. The well-established healthcare infrastructures in these regions facilitate the efficient adoption of advanced IV tubing systems and accessories.
Product Insights
Primary IV tubing sets led the market with a revenue share of 32.2% in 2024. Primary IV tubing sets are vital in acute and chronic care settings, facilitating the rapid delivery of lifesaving medications and supporting long-term treatments such as chemotherapy. Their continuous design advancements and cost-effectiveness make them crucial, enabling hospitals and clinics to procure in bulk without financial strain, ensuring availability for diverse medical procedures.
Extension IV tubing sets are expected to grow at the fastest CAGR of 5.8% over the forecast period. Extension IV tubing sets enhance patient maneuverability in critical care and surgical environments. In ICUs, they enable efficient management of multiple IV lines while minimizing entanglement risks. Innovations from companies such as ICU Medical and Smiths Medical incorporate advanced materials and safety features, such as anti-reflux valves and needleless connectors, to reduce infection and injury risks.
Application Insights
Peripheral intravenous catheter insertion dominated the market with a revenue share of 48.5% in 2024, driven by its extensive application across various medical settings and the rising adoption of minimally invasive procedures. Recent technological advancements have yielded improved biocompatible designs that enhance patient safety and comfort, incorporating features such as closed-system configurations, needleless connectors, and anti-reflux valves to reduce the risks of infections and needle-stick injuries.
Central venous catheter (CVC) placement is expected to register the fastest CAGR of 5.4% over the forecast period, owing to the essential role of central venous catheters (CVCs) in managing acute and chronic medical conditions, alongside the increasing demand for long-term intravenous therapy. For example, patients with chronic kidney disease and those needing long-term parenteral nutrition rely on tunneled or non-tunneled CVCs for reliable vascular access.
End-use Insights
Hospitals held the largest market share of 45.1% in 2024 due to the high volume of surgical procedures, the prevalence of chronic diseases, and the availability of advanced healthcare infrastructure and specialized medical personnel. Hospitals play a crucial role in managing complex cases, resulting in heightened demand for intravenous tubing sets and accessories for anesthesia and medication administration.
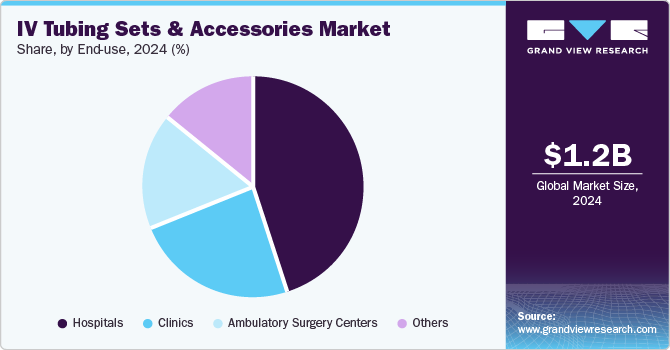
Ambulatory surgical centers (ASCs) are projected to the fastest growth of 5.8% over the forecast period. are gaining popularity for their cost-effectiveness and convenience, allowing outpatient procedures that previously required hospital stays. This transition reduces healthcare costs, enhances patient turnover, and optimizes hospital resources for critical cases, thereby increasing the demand for intravenous tubing sets for anesthesia, fluids, and medication administration in ASCs.
Regional Insights
North America IV tubing sets and accessories market dominated the global market with a revenue share of 46.9% in 2024. Market growth in the region is driven by the high prevalence of chronic diseases, an aging demographic, technological advancements, and an expansion of home healthcare services, influenced by the COVID-19 pandemic. Approximately 14,714 urgent care centers in the U.S. provide care to over 100 million patients annually, supported by stringent FDA regulations.
U.S. IV Tubing Sets And Accessories Market Trends
The IV tubing sets and accessories market in U.S. dominated the North America IV tubing sets & accessories market with a revenue share of 83.5% in 2024. As the population ages, the demand for effective and reliable intravenous treatments is increasing, driving market growth. According to the National Library of Medicine, nearly nine major surgeries were performed per 100 older individuals in the U.S. in July 2021, highlighting the need for high-quality IV tubing sets amidst ongoing medical technology advancements.
Europe IV Tubing Sets And Accessories Market Trends
Europe IV tubing sets and accessories market held substantial market share in 2024. The region is defined by advancements in medical technology, demographic shifts, and evolving healthcare practices. The European Medicines Agency (EMA) and national regulatory bodies implement stringent standards for the safety and efficacy of medical devices, such as IV tubing sets, fostering trust among healthcare providers and patients while driving market growth.
Asia Pacific IV Tubing Sets And Accessories Market Trends
Asia Pacific IV tubing sets and accessories market is expected to register the fastest CAGR of 5.7% in the forecast period, driven by its rapidly expanding population and rising prevalence of chronic diseases, such as diabetes, cancer, and cardiovascular conditions, driving demand for intravenous therapies. Economic development in countries such as China and India is also escalating healthcare expenditures, further enhancing the market potential.
Key IV Tubing Sets And Accessories Company Insights
Some key companies operating in the market include B. Braun Medical Inc.; ICU Medical, Inc.; Baxter; among others. Strategic initiatives encompass mergers, partnerships, and product launches, while companies are also increasing investments in research and development to strengthen market presence.
-
ICU Medical, Inc. specializes in innovative infusion therapy solutions, offering IV tubing sets equipped with advanced safety features. The company's designs aim to minimize infection risks and medication errors, thereby supporting healthcare professionals in providing effective patient care.
-
Baxter offers varied IV tubing sets, including primary, secondary, and extension configurations. The company’s products enhance patient care by ensuring reliable delivery of medications and fluids in clinical settings.
Key IV Tubing Sets And Accessories Companies:
The following are the leading companies in the IV tubing sets and accessories market. These companies collectively hold the largest market share and dictate industry trends.
- B. Braun Medical Inc.
- ICU Medical, Inc.
- Baxter
- BD
- Fresenius Kabi AG
- Zyno Medical (Intuvie Holdings LLC)
- Nipro Medical Corporation
- Polymedicure
Recent Developments
-
In September 2024, B. Braun Medical received FDA 510(k) clearance for the Introcan Safety 2 Deep Access IV Catheter, enhancing vascular access with improved dwell times and advanced safety features.
-
In August 2024, ICU Medical obtained FDA 510(k) clearance for the Plum Duo infusion pump and LifeShield infusion safety software, enhancing IV medication safety, accuracy, and efficiency in healthcare settings.
-
In July 2024, B. Braun Medical announced the first U.S. conversion to NRFit Connectors at a Midwest children’s hospital, enhancing patient safety by preventing misconnections in neuraxial anesthesia applications.
IV Tubing Sets And Accessories Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 1.29 billion
Revenue forecast in 2030
USD 1.65 billion
Growth rate
CAGR of 5.1% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, trends
Segments covered
Product, application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, India, Australia, South Korea, Thailand, Brazil, Argentina, South Africa, Saudi Arabia, UAE, Kuwait
Key companies profiled
B. Braun Medical Inc.; ICU Medical, Inc.; Baxter; BD; Fresenius Kabi AG; Zyno Medical (Intuvie Holdings LLC); Nipro Medical Corporation; Polymedicure
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
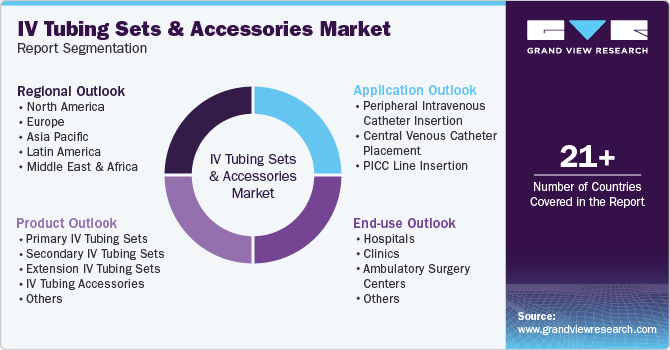
Global IV Tubing Sets And Accessories Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global IV tubing sets and accessories market report based on product, application, end-use, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Primary IV Tubing Sets
-
Secondary IV Tubing Sets
-
Extension IV Tubing Sets
-
IV Tubing Accessories
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Peripheral Intravenous Catheter Insertion
-
Central Venous Catheter Placement
-
PICC Line Insertion
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Clinics
-
Ambulatory Surgery Centers
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Some key players operating in the IV tubing sets & accessories market include, Baxter, B. Braun Melsungen AG, Fresenius Kabi AG, and ICU Medical, Inc.
b. Key factors that are driving the market growth include, increasing government initiatives, rising natality rate, risk of malnutrition, and increasing incidence of cancer are anticipated to drive the growth of the market over the forecast period.
b. The global IV tubing sets & accessories market size was estimated at USD 1.24 billion in 2024 and is expected to reach USD 1.29 billion in 2025.
b. The global IV tubing sets & accessories market is expected to grow at a compound annual growth rate of 5.1% from 2025 to 2030 to reach USD 1.65 billion by 2030.
b. The peripheral intravenous catheter insertion market dominated the IV tubing sets & accessories market with a share of over 48.00% in 2024. This is attributable to the technological advancement, increasing use due to its several advantages, and rising number of surgeries
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










