- Home
- »
- Biotechnology
- »
-
Lipid Nanoparticle Market Size & Share, Industry Report 2030GVR Report cover
![Lipid Nanoparticle Market Size, Share & Trends Report]()
Lipid Nanoparticle Market (2025 - 2030) Size, Share & Trends Analysis Report By LNP (Liposomes, Nanostructured Lipid Carriers), By Molecule (mRNA, siRNA), By Application, By Indication, By End Use, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-547-5
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Lipid Nanoparticle Market Summary
The global lipid nanoparticle market size was estimated at USD 786.4 million in 2024 and is projected to reach USD 1,541.6 million by 2030, growing at a CAGR of 13.64% from 2025 to 2030. The market growth is driven by factors such as the rising demand for drugs utilizing lipid nanoparticles (LNPs) as delivery systems, the increasing prevalence of chronic diseases such as cancer, cardiovascular conditions, and autoimmune disorders, and ongoing technological advancements in RNA-based therapies.
Key Market Trends & Insights
- North America accounted for 37.49% of the global market share in 2024.
- The U.S. held the largest share in 2024 in the North America region.
- By molecule, the mRNA segment dominated market with the largest revenue share of 54.98% in 2024.
- By application, the therapeutics segment dominated market with the largest revenue share of 61.90% in 2024.
- By indication, the cancer segment held the largest market share of 48.31% in 2024.
Market Size & Forecast
- 2024 Market Size: USD 786.4 Million
- 2030 Projected Market Size: USD 1,541.6 Million
- CAGR (2025-2030): 13.64%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
In addition, the growing number of joint ventures and partnerships among industry players is expected to support market expansion further. For instance, in July 2023, Lawrence Berkeley National Laboratory partnered with Genentech, Inc. (a Roche Group member) to develop more effective LNPs for drug delivery, fostering innovation through collaborative research.The COVID-19 pandemic acted as a pivotal accelerator for the LNP industry. The success of mRNA-based vaccines, such as Pfizer-BioNTech's Comirnaty and Moderna's Spikevax, demonstrated the crucial role of LNPs in safely and effectively delivering mRNA into human cells. This validation of LNP technology on a global scale not only highlighted its potential but also spurred a wave of new research and commercial interest. As a result, many biopharmaceutical companies began exploring LNPs for a broader range of vaccine and therapeutic applications, leading to increased investments, faster regulatory pathways, and enhanced public-private partnerships during and after the pandemic.

Government funding plays a vital role in advancing research and development efforts focused on innovative lipid nanoparticle (LNP)-based products, particularly in drug delivery applications across various therapeutic areas. In addition, increased R&D investments by leading market players are fueling growth by encouraging innovation, broadening application scope, and driving technological progress. For instance, in June 2022, Evonik announced a USD 220 million investment to build a large-scale pharmaceutical lipid production facility in the U.S., with up to USD 150 million in funding support from the Biomedical Advanced Research and Development Authority (BARDA).
Moreover, ongoing innovation in LNP formulation techniques is propelling the development of next-generation delivery systems with enhanced stability, improved drug-loading efficiency, and targeted delivery capabilities. This, in turn, increases the demand for advanced raw materials that support the creation of these novel LNPs. For example, in December 2023, researchers from the Leslie Dan Faculty of Pharmacy at the University of Toronto developed a new ionizable lipid nanoparticle that effectively delivers mRNA to muscle tissue while minimizing off-target effects. Their study showed that this approach induced strong immune responses at the cellular level, highlighting the potential of cutting-edge LNP technologies to reshape drug delivery.
LNPs in the Commercial Market and Clinical Trials
LNP subgroup
Active substance
Disease/applications
Products
liposomes
doxorubicin/daunorubicin
cancer
Doxil, Myocet, Vixeos, DaunoXome, Transdrug
paclitaxel
cancer
Abraxane
Solid lipid nanoparticles
mitoxantrone
hepatocarcinoma
Mitoxantrone-loaded polybutylcyanacrylate nanoparticles (DHAD-PBCA-NPs) (phase II clinical trial)
doxorubicin
hepatocarcinoma
Doxorubicin Transdrug (DT) (Phase III clinical trial)
nanostructured lipid carriers
acitretin
psoriasis
Acitretin Precirol ATO 5/oleic acid/Tween 80 (Randomized Controlled Trial)
mRNA-1273
COVID-19
Spikevax
lipid polymer hybrid nanoparticle
docetaxel
pancreatic cancer
Docetaxel polymeric nanoparticle (phase I clinical trial)
docetaxel
lung cancer with KRAS mutation
BIND-014 (Docetaxel Nanoparticles for Injectable Suspension) (phase II clinical trial)
Source: ACS Publications
Furthermore, the rising global burden of cancer is significantly impacting the demand for LNP in the pharmaceutical and biotech industries. According to the American Cancer Society, the U.S. was expected to see 2,001,140 new cancer cases and 611,720 related deaths in 2024 alone. As cancer incidence increases, so does the need for effective and targeted treatment options. LNPs offer a promising delivery platform for anticancer drugs by enhancing solubility, protecting therapeutic payloads, and improving delivery to tumor sites. This growing demand is accelerating the need for high-quality raw materials used in LNP synthesis, further driving the lipid nanoparticle industry forward.
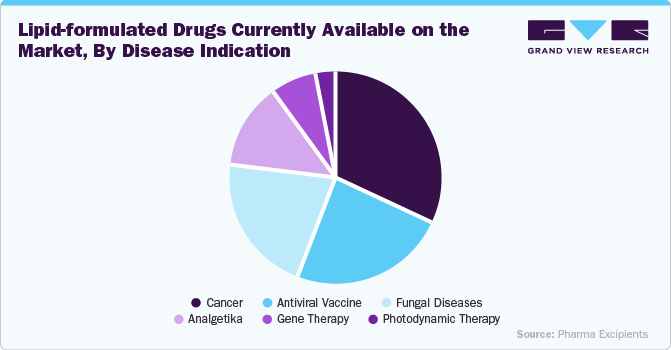
Lipid-based drugs on the market address a wide range of disease indications, leveraging their advanced delivery capabilities to enhance treatment outcomes. In oncology, liposomal formulations such as Doxil (doxorubicin) and Onivyde (irinotecan) are used to improve drug targeting and reduce toxicity. For infectious diseases, lipid-based antifungals like AmBisome (amphotericin B) and liposomal formulations for tuberculosis and hepatitis have shown improved efficacy. In the field of vaccines, lipid nanoparticle-based mRNA vaccines like Comirnaty (Pfizer-BioNTech) and Spikevax (Moderna) have revolutionized prevention strategies for COVID-19. In addition, in rare genetic disorders, lipid-based delivery is used for siRNA therapies like Onpattro (patisiran) for hereditary transthyretin-mediated amyloidosis, underscoring the versatility of lipid-based drugs across diverse therapeutic areas.
Market Concentration & Characteristics
The lipid nanoparticle industry is witnessing a surge in innovation driven by advances in formulation techniques, tailored lipid design, integration of RNA-based therapeutics, and scalable manufacturing processes. These innovations are revolutionizing drug delivery across diverse therapeutic areas, from infectious diseases to cancer and genetic disorders. As research continues to grow the boundaries of LNP technology, stakeholders must collaborate to accelerate the translation of these innovations into clinically impactful therapies and improve patient outcomes.
The lipid nanoparticle industry has seen a significant level of merger and acquisition (M&A) activity in recent years. This can be attributed to the growing demand for LNP in the pharmaceutical industry, which has become a key driver for market growth. The advancements in LNP technology have been a primary driver of M&A activity. As companies strive to enhance their capabilities in formulation development, manufacturing processes, and scalability, acquiring specialized firms with proprietary technologies or expertise becomes an attractive strategy, which is expected to fuel further growth in the market.
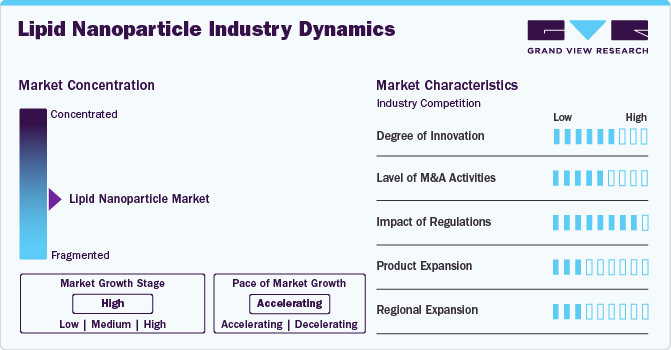
The impact of regulations on the market can be significant. Regulations play a significant role in the lipid nanoparticle industry. The regulatory framework governs the manufacturing, testing, and distribution of lipid nanoparticle-based drug delivery systems. The regulatory bodies ensure the safety, efficacy, and quality of these products and also monitor the compliance of the industry players with the regulatory guidelines. Stringent regulations can increase the time and costs associated with the development and commercialization of lipid nanoparticle-based drug delivery systems.
The lipid nanoparticle industry has witnessed significant product expansion trends in recent years. With the increasing demand for advanced drug delivery systems, industry players have been focusing on developing new and innovative products to cater to the evolving needs of the market. Some of the key product expansion trends in the market include the development of new lipid-based drug delivery systems, the use of biodegradable polymers in nanoparticle formulations, and the integration of advanced technologies such as nanotechnology and microfluidics in the manufacturing process. These product expansion trends are expected to drive the growth of the market and create new opportunities for industry players in the coming years.
The concentration of regional expansion in the market can vary by region, and it is influenced by factors such as research funding, healthcare policies, and the level of technological adoption in different sectors. Market dynamics also shift based on emerging applications and advancements in LNP technologies.
LNP Insights
The liposomes segment dominated the overall market, with the largest revenue share of 49.98% in 2024. Liposomes offer biocompatibility, the ability to encapsulate both hydrophilic and lipophilic drugs, and improved pharmacokinetics, making them ideal carriers for chemotherapeutics, vaccines, and antifungal agents. The increasing demand for targeted and controlled-release drug delivery systems, along with rising R&D investments in nanomedicine, is further fueling the adoption of liposome-based formulations.
The nanostructured lipid carriers (NLCs) segment is expected to grow at the fastest CAGR during the forecast period. NLCs offer enhanced drug loading capacity, improved stability, and controlled release profiles, making them highly suitable for delivering poorly water-soluble drugs. Their applications span across pharmaceuticals, cosmetics, and nutraceuticals, with increasing demand for non-invasive and patient-friendly delivery systems. Rising interest in personalized medicine and advancements in lipid-based formulation technologies further support the adoption of NLCs, especially in oncology, dermatology, and chronic disease management.
Molecule Insights
The mRNA segment dominated the overall market with the largest revenue share of 54.98% in 2024 and is expected to grow at the fastest CAGR during the forecast period. LNPs are essential for the safe and effective delivery of mRNA, protecting it from degradation and facilitating cellular uptake. The flexibility and rapid development timeline of mRNA platforms have encouraged their exploration in a wide range of applications, including infectious diseases, oncology, and rare genetic disorders. As pharmaceutical companies and biotech firms increasingly invest in mRNA therapeutics, the demand for optimized LNP delivery systems continues to rise, fueling market growth.
The siRNA segment is expected to grow at a significant CAGR during the forecast period. siRNA-based treatments require efficient and targeted delivery systems to reach specific cells and tissues without degradation, making LNPs an ideal carrier due to their ability to encapsulate and protect these delicate molecules. The success of approved siRNA drugs like Onpattro (patisiran) has validated the potential of LNP-based delivery for RNA interference (RNAi) therapies. As research in genetic diseases, cancer, and rare disorders advances, the demand for LNPs tailored for siRNA delivery continues to expand, propelling market growth.
Application Insights
The therapeutics segment dominated the overall market, with the largest revenue share of 61.90% in 2024. LNP is widely used in the pharmaceutical industry for drug delivery due to its ability to encapsulate and deliver therapeutic agents to specific targets in the body. As the demand for novel drug delivery systems grows, it is expected to increase the demand for lipid nanoparticles. With ongoing advancements in nanotechnology and nanomedicine, there is a growing focus on developing lipid-based nanoparticles for targeted drug delivery. These advancements are driving the demand for LNP in therapeutics.
The research segment is expected to grow at the fastest CAGR during the forecast period due to the increasing demand for efficient drug delivery systems and the use of lipid nanoparticles in various research activities. Lipid nanoparticles are widely used in the development of novel drug formulations, as they possess several advantages, such as biocompatibility, high drug loading capacity, and controlled release of drugs. Moreover, the rising prevalence of chronic diseases such as cancer and the need for targeted drug delivery has further boosted the demand for LNP in research applications.
Indication Insights
The cancer segment held the largest market share of 48.31% in 2024 and is expected to grow at the fastest CAGR over the forecast period. LNP is used as drug delivery to target cancer cells and increase the efficacy of treatment. The raw materials required to produce these nanoparticles include lipids, surfactants, and polymers. According to WHO, it is estimated that the number of new cancer cases is expected to exceed 35 million by the year 2050, showing a significant increase of 77% from the estimated number of cases, which was 20 million in 2022. The rising cancer burden and the potential of LNPs in cancer treatment are driving significant research and development efforts in this area. This R&D activity is fueling the demand for LNP raw materials, including lipids, polymers, and other components.
The infectious diseases segment is anticipated to grow at a significant CAGR over the forecast period. Lipid nanoparticles are used in the development of mRNA vaccines, which have become a key tool in the fight against COVID-19. The LNP helps protect the mRNA from degradation in the body and facilitates its delivery to cells. As more countries roll out vaccination programs, the demand for LNP has increased significantly. In addition to COVID-19, lipid nanoparticles are also being studied for the treatment of other infectious diseases, such as HIV and influenza. This has further contributed to the growth of the market.
End Use Insights
In terms of end use, pharmaceutical & biotechnology companies held the largest market share of 57.76% in 2024. Pharmaceutical and biotechnology companies use LNPs to develop advanced drug delivery systems with improved efficacy, safety, and specificity for various therapeutic applications, including cancer treatment, gene therapy, and personalized medicine. The demand for Lipid nanoparticles has risen significantly as pharmaceutical and biotechnology companies seek reliable and high-quality sources to manufacture their products.

The academic & research institutes segment is expected to grow at the fastest CAGR over the forecast period. Academic and research institutes play a crucial role in driving the growth of the lipid nanoparticles (LNP) market by advancing fundamental research and innovation in drug delivery technologies. These institutions are at the forefront of developing next-generation LNP formulations for various therapeutic applications, including mRNA vaccines, gene therapy, and targeted cancer treatments. Increased government and private funding for translational research, along with collaborations between academia and industry, have accelerated the discovery of novel lipid structures and improved delivery mechanisms.
Regional Insights
North America lipid nanoparticle industry accounted for 37.49% of the global market share in 2024. The presence of a robust pharmaceutical and biotechnology industry in North America contributes to the growth of the market. Pharmaceutical companies and biotech firms in the U.S. and Canada invest heavily in research and development, driving demand for lipid nanoparticle-based drug delivery systems. The shift towards biologics and gene therapy products in North America has created opportunities for lipid nanoparticle-based delivery systems. Lipid nanoparticles are utilized for the delivery of nucleic acids, including mRNA, siRNA, and gene-editing tools, for the treatment of various diseases. The approval of mRNA vaccines for COVID-19 has further highlighted the potential of lipid nanoparticles in gene therapy.
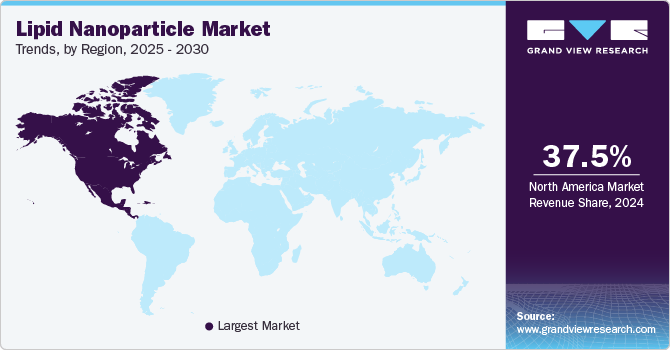
U.S. Lipid Nanoparticle Market Trends
The lipid nanoparticle industry in the U.S. held the largest share in 2024 in the North America region. Pharmaceutical companies, biotechnology firms, and research institutions in the U.S. are investing heavily in R&D efforts focused on lipid nanoparticle-based therapies. This investment is driving innovation in lipid nanoparticle formulations, manufacturing processes, and applications, thereby expanding the market. For instance, in June 2022, Gattefossé announced the construction of its new production facility in Texas, U.S., with an investment of USD 50 million. The plant is expected to be operational in 2024.
Europe Lipid Nanoparticle Market Trends
Europe lipid nanoparticle industry is expected to grow over the forecast period. Europe has a robust pharmaceutical and biotechnology industry that is continuously seeking innovative drug delivery systems to improve therapeutic outcomes. Lipid nanoparticles offer advantages such as enhanced drug solubility, stability, and targeted delivery, making them increasingly attractive to pharmaceutical companies developing novel therapeutics. Collaboration between academia, industry, and research institutions is common in Europe, facilitating interdisciplinary research and technology transfer. Collaborative initiatives aimed at advancing lipid nanoparticle technologies, such as Horizon 2020 projects and public-private partnerships, contribute to market expansion by fostering innovation and knowledge exchange.
The lipid nanoparticle industry in the UK held a significant share in 2024. The increasing prevalence of chronic diseases such as cancer and cardiovascular diseases has led to a growing need for more effective therapies. In addition, the UK government has been investing heavily in the development of advanced drug delivery systems, including lipid nanoparticles, which have also contributed to the growth of the market.
The lipid nanoparticle industry in France held a significant share in 2024. France has a well-established regulatory framework for the approval and commercialization of pharmaceutical products. Regulatory agencies such as the French National Agency for Medicines and Health Products Safety (ANSM) ensure product safety and efficacy, which enhances investor confidence and supports market growth. France faces healthcare challenges such as an aging population, increasing prevalence of chronic diseases, and rising healthcare costs. Lipid nanoparticles offer advantageous solutions for addressing these challenges by improving drug delivery efficiency, reducing side effects, and enhancing therapeutic outcomes. The growing demand for innovative healthcare solutions drives the growth of the market in France.
The lipid nanoparticle industry in Germany held a significant revenue share in 2024. Germany boasts a leading pharmaceutical industry renowned for its research and development capabilities. This strong foundation fuels a high demand for innovative drug delivery solutions, including LNPs, for various therapeutic applications. The German government actively supports advancements in LNP technologies through funding and policy initiatives. It includes the "Biotech 2030" strategy and the "Clusters4Future" program, fostering a stimulating environment for research, development, and commercialization of LNP-based products, ultimately driving the raw materials market.
Asia Pacific Lipid Nanoparticle Market Trends
The Asia Pacific lipid nanoparticle industry is expected to exhibit the fastest CAGR over the forecast period. The region is experiencing rapid economic growth, leading to higher healthcare expenditure and investments in medical research and development. As healthcare infrastructure improves and access to healthcare services expands, there is a growing demand for advanced drug delivery systems, including lipid nanoparticles, driving market growth. Furthermore, governments in the Asia Pacific region are increasingly supporting research and development initiatives in the life sciences sector through grants, subsidies, and incentives. For instance, initiatives such as China's "Made in China 2025" plan and India's "Biotechnology Industry Research Assistance Council" (BIRAC) aim to promote innovation and entrepreneurship in biotechnology. Such support encourages investment in lipid nanoparticle research and development, contributing to market growth.
The lipid nanoparticle industry in China held a significant regional market share in 2024. Cancer is a significant driver of the market in China. Lipid nanoparticles are utilized for the delivery of chemotherapy drugs, RNA-based therapeutics, and imaging agents for cancer diagnosis and treatment monitoring. The ability of lipid nanoparticles to encapsulate and target cancer drugs to tumor cells while minimizing systemic toxicity is particularly valuable in cancer therapy. In addition, China faces various infectious disease challenges, including viral infections such as hepatitis, influenza, and COVID-19, as well as bacterial infections. Lipid nanoparticles are being investigated for the delivery of antiviral drugs, vaccines, and RNA-based therapeutics for infectious diseases. The development of lipid nanoparticle-based COVID-19 vaccines and treatments has further accelerated the growth of this market segment in China.
The lipid nanoparticle industry in Japan held a significant share in 2024. It is driven by the increasing adoption of lipid nanoparticles in drug delivery systems due to their enhanced bioavailability, biocompatibility, and controlled drug release properties. In addition, the rising prevalence of chronic diseases such as cancer and cardiovascular diseases has led to an increase in demand for lipid nanoparticle-based therapies in the country. Furthermore, government initiatives to promote R&D activities in the field of nanotechnology are also expected to boost the growth of the market in Japan.
Middle East & Africa Lipid Nanoparticle Market Trends
The lipid nanoparticles (LNP) industry in the Middle East & Africa is primarily driven by growing government initiatives to improve healthcare infrastructure, increasing prevalence of chronic diseases such as cancer and infectious disorders, and the rising adoption of advanced drug delivery systems. Countries such as Saudi Arabia, the UAE, and South Africa are investing in biotechnology and pharmaceutical R&D, encouraging local manufacturing and collaborations with global players. In addition, the region’s focus on pandemic preparedness and vaccine development, influenced by the success of mRNA-LNP COVID-19 vaccines, is fostering interest in lipid nanoparticle technologies for broader therapeutic applications.
The lipid nanoparticle industry in Saudi Arabia is driven primarily due to factors such as the increasing focus on research and development activities, the growing demand for advanced drug delivery systems, and the rising prevalence of chronic diseases. In addition, the increasing investments in the healthcare sector and the growing awareness about the benefits of LNP in drug delivery are also driving the growth of this market in Saudi Arabia. The market is expected to continue its growth in the coming years due to favorable government initiatives and policies aimed at promoting the adoption of advanced healthcare technologies.
The lipid nanoparticle industry in Kuwait held a moderate revenue share in 2024. Kuwait market is expected to grow over the forecast period. It is driven by factors such as the increasing demand for advanced drug delivery systems, the rising prevalence of chronic diseases, growing investment in research and development activities, and the rising adoption of nanotechnology in the healthcare sector. In addition, the increasing geriatric population and benefits of LNP in drug delivery are also contributing to the growth of this market.
Key Lipid Nanoparticle Company Insights
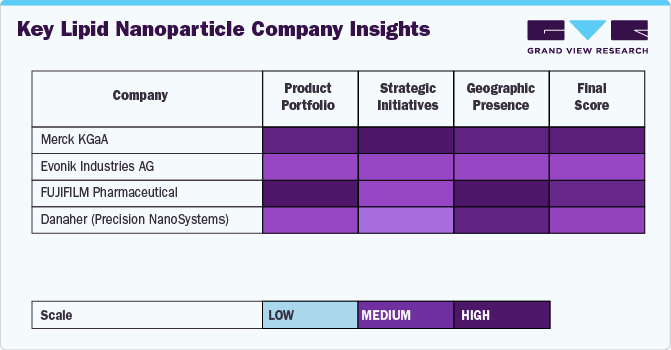
The market players operating in the lipid nanoparticle industry are adopting product approval to increase the reach of their products in the market and improve the availability of their products & services in diverse geographical areas, along with expansion as a strategy to enhance production/research activities. In addition, several market players are acquiring smaller players to strengthen their market position. This strategy enables companies to increase their capabilities, expand their product portfolios, and improve their competencies.
Key Lipid Nanoparticle Companies:
The following are the leading companies in the lipid nanoparticle market. These companies collectively hold the largest market share and dictate industry trends.
- Merck KGaA
- Evonik Industries AG
- Gattefosse
- FUJIFILM Pharmaceutical
- Danaher (Precision NanoSystems)
- Creative Biolabs
- IOI Oleo GmbH
- NOF Corporation
- Lipoid GmbH
- Cyman Chemical
Recent Development
-
In August 2024, Evonik enhanced its formulation capabilities for lipid nanoparticles utilized in mRNA and gene therapy applications.
-
In July 2023, Cytiva introduced a formulation system designed to facilitate smooth end-to-end manufacturing of lipid nanoparticle medicines across clinical and commercial stages. This system simplifies the process, enabling efficient and comprehensive production of lipid nanoparticle-based medications from development through to commercialization.
-
In July 2023, ModernaTX, Inc. and McGill University announced the collaboration. The collaboration is focused on two new projects focused on LNP research-specific aspects of LNP properties, potentially their characteristics or comparison to naturally occurring particles.
Global Lipid Nanoparticle Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 813.4 million
Revenue forecast in 2030
USD 1,541.6 million
Growth rate
CAGR of 13.64% from 2025 to 2030
Actual data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
LNP, molecule, indication, application, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand;South Korea; Brazil; Argentina; South Africa; Saudi Arabia, UAE; Kuwait
Key companies profiled
Merck KGaA; Evonik Industries AG; Gattefosse; FUJIFILM Pharmaceutical; Danaher (Precision NanoSystems); Creative Biolabs; IOI Oleo GmbH; NOF Corporation; Lipoid GmbH; Cyman Chemical
Customization scope
Free report customization (equivalent up to 8 analyst's working days) with purchase. Addition or alteration to country, regional & segment scope.
Global Lipid Nanoparticle Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the global lipid nanoparticle market based on LNP, molecule, indication, application, product, indication, end use, and region.
-
LNP Outlook (Revenue, USD Million, 2018 - 2030)
-
Liposomes
-
Solid lipid nanoparticles
-
Nanostructured lipid carriers
-
Others
-
-
Molecule Outlook (Revenue, USD Million, 2018 - 2030)
-
siRNA
-
mRNA
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Therapeutics
-
Research
-
-
Indication Outlook (Revenue, USD Million, 2018 - 2030)
-
Cancer
-
Infectious Diseases
-
Blood Diseases
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Pharmaceutical & Biotechnology Companies
-
Academic & Research Institutes
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global lipid nanoparticle market size was estimated at USD 786.4 million in 2024 and is expected to reach USD 813.4 million in 2025.
b. The global lipid nanoparticle market is expected to grow at a compound annual growth rate of 13.64% from 2025 to 2030 to reach USD 1,541.6 million by 2030.
b. North America accounted for 37.49% of market share in 2024. The presence of a robust pharmaceutical and biotechnology industry in North America contributes to the growth of the lipid nanoparticle market. Pharmaceutical companies and biotech firms in the U.S. and Canada invest heavily in research and development, driving demand for lipid nanoparticle-based drug delivery systems.
b. Some key players operating in the lipid nanoparticle market include Merck KGaA, Evonik Industries AG, Gattefosse, FUJIFILM Pharmaceutical, Danaher (Precision NanoSystems), Creative Biolabs, IOI Oleo GmbH, NOF Corporation, Lipoid GmbH, Cyman Chemical
b. The market growth is driven by factors such as the rising demand for drugs utilizing lipid nanoparticles (LNPs) as delivery systems, the increasing prevalence of chronic diseases like cancer, cardiovascular conditions, and autoimmune disorders, and ongoing technological advancements in RNA-based therapies.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










