- Home
- »
- Pharmaceuticals
- »
-
Lymphoma Treatment Market Size And Share Report, 2030GVR Report cover
![Lymphoma Treatment Market Size, Share & Trends Report]()
Lymphoma Treatment Market (2025 - 2030) Size, Share & Trends Analysis Report By Type (Hodgkin Lymphoma, Non-Hodgkin Lymphoma), By Drugs (Adcetris, Opdivo, Rituxan, Imbruvica, Keytruda), By Region, And Segment Forecasts
- Report ID: GVR-3-68038-983-8
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Lymphoma Treatment Market Summary
The global lymphoma treatment market size was valued at USD 7.41 billion in 2024 and is projected to reach USD 11.97 billion by 2030, growing at a CAGR of 8.3% from 2025 to 2030. The market growth is attributed to the increasing incidence of lymphoma, particularly non-Hodgkin lymphoma, which has heightened demand for effective therapies.
Key Market Trends & Insights
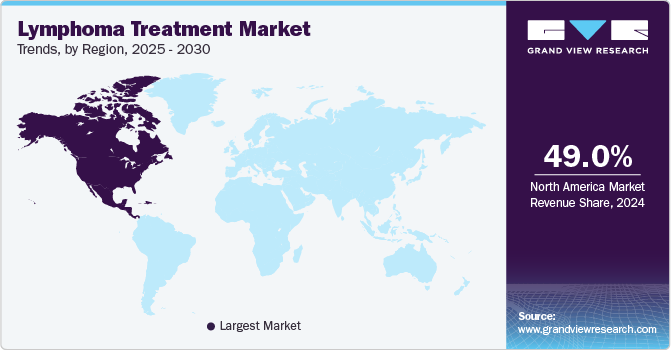
- North America dominated the global market and accounted for the largest revenue share of 49.0% in 2024.
- The U.S. lymphoma treatment market dominated North America with the largest revenue share in 2024.
- By type, non-hodgkin lymphoma (NHL) segment dominated the market with the largest revenue share of 86.2% in 2024.
- By drug, rituxan (MabThera) segment dominated the market and accounted for the largest revenue share of 28.8% in 2024.
- By distribution channel, hospital pharmacies segment dominated the market and accounted for the largest revenue share of 36.1% in 2024.
Market Size & Forecast
- 2024 Market Size: USD 7.41 Billion
- 2030 Projected Market Size: USD 11.97 Billion
- CAGR (2025-2030): 8.3%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
In addition, advancements in treatment options, including targeted therapies and immunotherapies, have significantly improved patient outcomes. Furthermore, strategic partnerships and a robust pipeline of novel therapies are expected to enhance market dynamics over the coming years.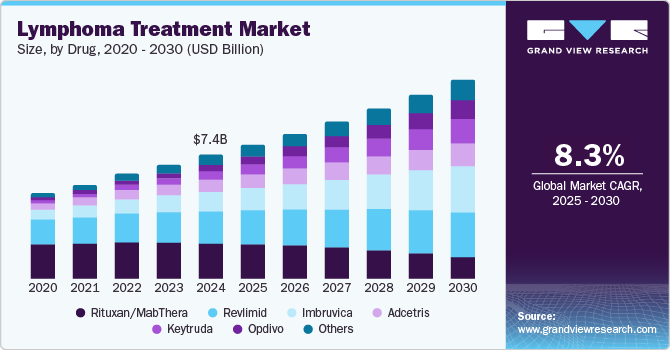
Lymphomas represent a significant portion of blood cancer cases, leading to a substantial number of individuals affected globally. The lack of early clinical symptoms often results in late-stage diagnoses, necessitating more aggressive treatment approaches for better outcomes. This urgency drives the demand for advanced therapeutic options, particularly for harder-to-treat lymphoma types. Prominent pharmaceutical companies are actively conducting clinical trials to develop innovative drug candidates to improve treatment efficacy and patient survival rates.
In addition, enhanced treatment strategies are crucial for patients with advanced disease, as they often require specialized therapies tailored to their specific conditions. Consequently, the need for cutting-edge treatments is expected to propel market growth during the forecast period. Furthermore, the increasing focus on research and development of new therapies, including immunotherapies and targeted treatments, reflects an ongoing commitment to addressing the unmet needs within this patient population. As healthcare systems strive to improve patient outcomes and reduce mortality rates associated with lymphoma, the market for lymphoma treatments is poised for significant expansion in the coming years.
Type Insights
Non-Hodgkin Lymphoma (NHL) dominated the market with the largest revenue share of 86.2% in 2024, driven by the increasing incidence of NHL, with projections indicating a significant rise in cases over the coming years. The availability of effective therapies, including targeted treatments and immunotherapies, has enhanced patient outcomes and fueled demand for advanced options. Furthermore, the presence of blockbuster drugs such as Rituxan and Imbruvica contributes to market expansion. Moreover, increasing healthcare expenditure and a focus on developing cost-effective solutions further support the growth of this segment in the lymphoma treatment market.
Hodgkin Lymphoma (HL) is expected to grow at a CAGR of 15.7% over the forecast period attributed to a rising number of diagnosed cases and advancements in diagnostic technologies that facilitate earlier detection. The introduction of new therapies and label extensions for existing treatments are expected to improve outcomes for patients with relapsed or refractory HL. Furthermore, a strong pipeline of innovative products is anticipated to drive market growth. Government initiatives and health awareness programs are also crucial in enhancing treatment accessibility and improving patient outcomes in Hodgkin lymphoma.
Drug Insights
Rituxan (MabThera) dominated the market and accounted for the largest revenue share of 28.8% in 2024, attributed to its established efficacy and broad usage across various indications, particularly non-Hodgkin lymphoma (NHL). Its high prescription rates in major markets such as the U.S. and Europe reflect its importance in treatment regimens. In addition, ongoing clinical trials and label extensions are expanding its applications, enhancing its market presence. The increasing prevalence of lymphoma and the demand for effective therapies further contribute to Rituxan's sustained growth in this competitive landscape.
Keytruda is expected to grow at the fastest CAGR of 18.9% over the forecast period, owing to its status as a leading immunotherapy option, particularly for advanced or refractory cases. The drug's expanding indications across multiple cancer types, including Hodgkin lymphoma, create opportunities for broader patient access. In addition, keytruda's combination therapies with other treatments are also being explored, enhancing therapeutic outcomes. Furthermore, ongoing clinical trials and increasing healthcare awareness contribute to its rising demand, positioning Keytruda as a critical player in the evolving landscape of lymphoma therapies.
Distribution Channel Insights
Hospital pharmacies dominated the market and accounted for the largest revenue share of 36.1% in 2024 attributed to the increasing prevalence of lymphoma cases requiring specialized care. Hospital pharmacies play a crucial role in dispensing complex therapies, including intravenous and newly developed drugs, ensuring patients receive appropriate medication under professional supervision. Furthermore, ongoing clinical trials and research initiatives conducted within hospitals enhance the availability of cutting-edge treatments, further fueling demand within this segment.
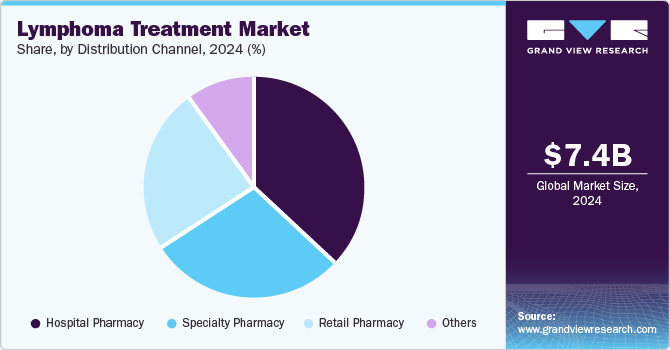
The specialty pharmacy segment is expected to grow at the fastest CAGR over the forecast period attributed to the growing demand for personalized and targeted therapies that require specialized handling and distribution. Specialty pharmacies focus on high-cost medications, including biologics and immunotherapies, which are increasingly used in lymphoma treatment regimens. Furthermore, the rising incidence of lymphoma and the introduction of novel therapeutics drive patients towards specialty pharmacies for access to advanced treatment options, thereby bolstering this segment's growth in the market.
Regional Insights
The lymphoma treatment market in North America dominated the global market and accounted for the largest revenue share of 49.0% in 2024, owing to the high prevalence of lymphoma cases, particularly non-Hodgkin lymphoma, which accounts for a significant portion of cancer diagnoses. In addition, leading pharmaceutical companies' constant research and development efforts further enhance the availability of cutting-edge treatments. Furthermore, favorable regulatory environments and numerous clinical trials contribute to the robust growth of this market.
U.S. Lymphoma Treatment Market Trends
The U.S. lymphoma treatment market dominated North America with the largest revenue share in 2024, owing to a rising incidence of lymphoma and advancements in treatment modalities such as immunotherapies and targeted therapies. The presence of major pharmaceutical companies conducting extensive R&D enhances the development pipeline for new therapies. Furthermore, increased public awareness about lymphoma and its treatment options encourages early diagnosis and intervention, which is crucial for effective management. Moreover, the high healthcare expenditure in the U.S. also supports adopting advanced therapeutic agents, driving market growth.
Asia Pacific Lymphoma Treatment Market Trends
The Asia Pacific lymphoma treatment market is projected to grow rapidly due to rising incidence rates and increasing healthcare spending. Countries in this region are experiencing improvements in healthcare infrastructure and access to innovative treatments. Introducing novel therapies and government initiatives to enhance cancer care are key drivers. Furthermore, growing public awareness about cancer prevention and treatment options is expected to lead to earlier diagnoses, thereby increasing demand for effective lymphoma treatments.
China Lymphoma Treatment Market Trends
The lymphoma treatment market in China is expected to grow at a significant CAGR over the forecast period, owing to its large population and increasing cancer incidence rates. The country has seen substantial investments in healthcare infrastructure and research initiatives to develop new therapies for lymphoma. In addition, regulatory authorities' approval of innovative drugs has also enhanced the treatment options available to patients. Furthermore, rising disposable incomes enable more patients to seek advanced treatments, further propelling market growth in China.
Europe Lymphoma Treatment Market Trends
The Europe lymphoma treatment market is expected to grow significantly over the forecast period, attributed to the high prevalence of lymphoma cases and a strong emphasis on research and development. In addition, the presence of well-established healthcare systems coupled with high patient awareness contributes to early diagnosis and effective management of lymphoma. Furthermore, collaborative efforts among pharmaceutical companies and regulatory bodies facilitate quicker approvals for new therapies, driving market growth across Europe.
Latin America Lymphoma Treatment Market Trends
The lymphoma treatment market in Latin America is expected to grow at a CAGR of 11.2% over the forecast period attributed to an increasing burden of lymphoma cases and rising healthcare investments. Although currently limited, the market is expected to expand as awareness about lymphoma increases among healthcare professionals and patients. In addition, efforts to improve healthcare infrastructure and access to advanced therapies are also contributing factors. Furthermore, collaborations between local governments and international pharmaceutical companies aim to enhance treatment options, which could significantly boost regional market growth.
Key Lymphoma Treatment Company Insights
Some of the key players in the lymphoma treatment market are F. Hoffmann-La Roche Ltd., AstraZeneca,Bayer AG,Novartis AG, and others. Key companies are adopting several strategies, such as investments in research and development to innovate and expand their product pipelines, focusing on advanced therapies such as immunotherapy and targeted treatments. In addition, collaborations and partnerships with research institutions enhance their capabilities in developing novel therapies. Furthermore, companies are pursuing mergers and acquisitions to strengthen their market position and diversify their offerings, ensuring they can meet the growing demand for effective lymphoma treatments.
-
Merck & Co., Inc. manufactures and markets several key products, including the immunotherapy Keytruda, which is widely used for treating different types of lymphoma, particularly in advanced stages. The company operates primarily in the oncology segment, focusing on targeted therapies and immunotherapies that enhance patient outcomes through precision medicine and robust clinical research initiatives.
-
F. Hoffmann-La Roche Ltd. manufactures various oncology products, including the monoclonal antibody Rituxan (MabThera), extensively used for treating non-Hodgkin lymphoma and other hematological malignancies. The company is heavily invested in the oncology segment, focusing on personalized medicine and innovative therapies that address unmet medical needs in lymphoma treatment through continuous research and development efforts.
Key Lymphoma Treatment Companies:
The following are the leading companies in the lymphoma treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Bristol-Myers Squibb Company
- Celgene Corporation
- Merck & Co., Inc.
- F. Hoffmann-La Roche Ltd.
- Seattle Genetics, Inc.
- Takeda Pharmaceutical Company Ltd.
- Johnson & Johnson
- Eli Lilly and Company
- Abbott Laboratories.
- AstraZeneca
- Bayer AG
- Novartis AG
Recent Developments
-
In May 2024, Bristol Myers Squibb announced that the U.S. Food and Drug Administration (FDA) had approved Breyanzi (lisocabtagene maraleucel), a CAR T-cell therapy, for treating adults with relapsed or refractory mantle cell lymphoma (MCL) after at least two prior therapies. This approval marks Breyanzi as the only CAR T-cell therapy available for four distinct subtypes of non-Hodgkin lymphoma, enhancing treatment options for patients with B-cell malignancies. Clinical trials showed an 85.3% response rate, offering hope for improved outcomes in this challenging lymphoma treatment landscape.
-
In May 2023, AbbVie announced that the U.S. Food and Drug Administration (FDA) had approved EPKINLY (epcoritamab-bysp), marking it as the first and only bispecific antibody for treating adult patients with refractory diffuse large B-cell lymphoma (DLBCL). This approval is based on the drug's efficacy in patients undergoing two or more prior therapies. Epcoritamab represents a significant advancement in lymphoma treatment, providing a new option for patients facing limited alternatives and enhancing AbbVie's commitment to improving outcomes in blood cancers.
Lymphoma Treatment Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 8.03 billion
Revenue forecast in 2030
USD 11.97 billion
Growth Rate
CAGR of 8.3% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, drug, distribution channel, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Germany, UK, France, Italy, Spain, China, Japan, India, South Korea, Australia, Brazil, Argentina, UAE, South Africa
Key companies profiled
Bristol-Myers Squibb Company; Celgene Corporation; Merck & Co., Inc.; F. Hoffmann-La Roche Ltd.; Seattle Genetics, Inc.; Takeda Pharmaceutical Company Ltd.; Johnson & Johnson; Eli Lilly and Company; Abbott Laboratories.; AstraZeneca; Bayer AG; Novartis AG.
Customization scope
Free report customization (equivalent to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Lymphoma Treatment Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and analyzes the latest industry trends in each sub-segment from 2018 to 2030. For this study, Grand View Research has segmented the global lymphoma treatment market report based on type, drug, distribution channel, and region.
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Hodgkin Lymphoma
-
Non-Hodgkin Lymphoma
-
-
Drug Outlook (Revenue, USD Million, 2018 - 2030)
-
Rituxan/MabThera
-
Revlimid
-
Imbruvica
-
Adcetris
-
Keytruda
-
Opdivo
-
Others
-
-
Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital Pharmacy
-
Retail Pharmacy
-
Specialty Pharmacy
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
UAE
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










