- Home
- »
- Pharmaceuticals
- »
-
Microneedle Flu Vaccine Market Size & Share Report, 2030GVR Report cover
![Microneedle Flu Vaccine Market Size, Share & Trends Report]()
Microneedle Flu Vaccine Market Size, Share & Trends Analysis Report, By Product Type (Solid Microneedle, Hollow Microneedle), By Vaccine Type, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-171-9
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Microneedle Flu Vaccine Market Trends
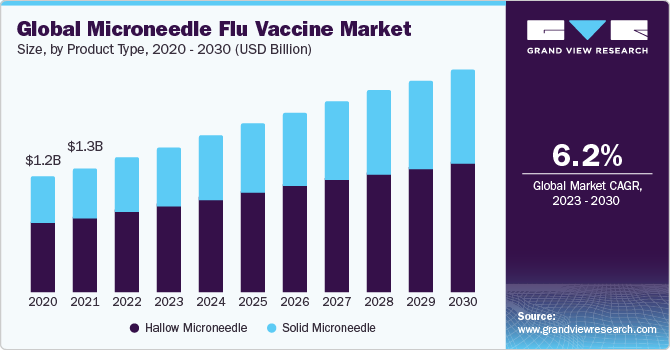
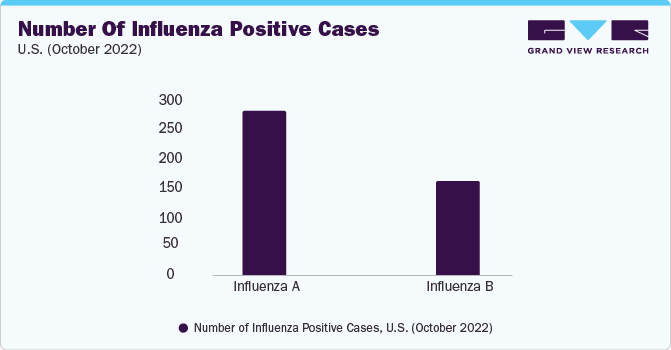
The global microneedle flu vaccine market size was valued at USD 1.4 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 6.20% from 2023 to 2030.The microneedle flu vaccine market is anticipated to experience significant growth due to the rising worldwide incidence of influenza and the concerted R&D initiatives undertaken by prominent industry players in the field of micro-needle flu vaccines. For instance, as per the Centers for Disease Control and Prevention (CDC) report published in 2022, between 2010 and 2020, the U.S. witnessed annual figures of 9 million flu cases, 12,000 to 52,000 fatalities due to influenza, and 140,000 to 710,000 hospitalizations underscoring the substantial impact of the disease.
Furthermore, patient convenience and acceptance are pivotal drivers for the market as they address a significant barrier to traditional needle-based vaccinations. Microneedle flu vaccines offer a less painful and less intimidating alternative, increasing patient willingness to receive vaccinations. This convenience factor is particularly vital for encouraging vaccine uptake in individuals who may have needle phobias or anxiety related to injections. By providing a more patient-friendly and non-invasive option, microneedle vaccines can promote higher vaccination rates and help achieve broader immunization coverage, ultimately contributing to public health by reducing the spread of the flu virus. In addition, microneedle delivery can potentially enhance the effectiveness of flu vaccines by more efficiently targeting the skin's immune cells, resulting in a stronger and potentially longer-lasting immune response against the flu virus. This increased effectiveness is a significant value proposition, as it not only offers better protection to individuals but also contributes to reduced disease transmission within communities.
Government initiatives play a crucial role in propelling the market by providing regulatory support, funding, and advocacy for innovative vaccination technologies. Governments often recognize the potential benefits of microneedle vaccines in terms of improved public health, safety, and pandemic preparedness. For instance, in 2021, Verndari, Inc. received a USD 1 million research and development grant from the U.S. Government Agency BARDA to expedite the advancement of VaxiPatch, a dermal patch vaccine technology with the potential to combat a range of infectious diseases, including influenza.
Product Type Insights
On the product type, the market is segmented intosolid microneedle and hollow microneedle. The hollow microneedle segment held the largest market share in 2022due to its capacity to directly inject the vaccine into the skin's dermal layer, where immune cells are more concentrated, thus potentially enhancing vaccine effectiveness. Hollow microneedles offer a well-established and precise method for vaccine delivery, ensuring that the vaccine reaches the target area with minimal loss or wastage. Their reliability in delivering the vaccine, along with a well-established safety profile, makes them the preferred choice for many healthcare providers and patients. On the other hand,solid microneedles are expected to show lucrative growth during 2023-2030.
Vaccine Type Insights
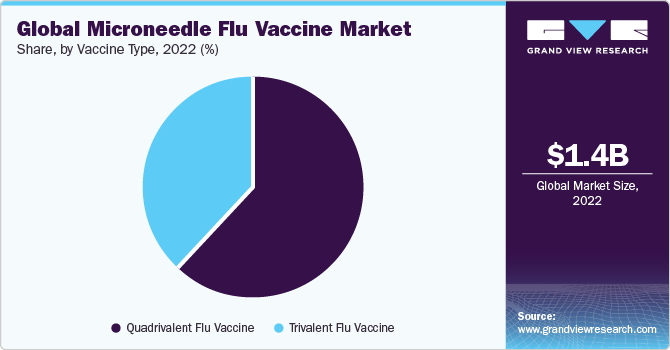
On the basis of vaccine type, the market is segmented into quadrivalent flu vaccine andtrivalent flu vaccine. The quadrivalent flu vaccine segment held the largest market share in 2022due to its broader coverage against multiple flu virus strains. Quadrivalent vaccines provide protection against two influenza A strains and two influenza B strains, offering a comprehensive defense against seasonal flu variations. This broader spectrum of protection is highly appealing to healthcare providers and patients, as it minimizes the risk of contracting a flu strain not covered by the vaccine. Quadrivalent flu vaccines, when combined with the convenience and effectiveness of microneedle delivery, make for a compelling choice, driving their dominance in the market and ensuring a higher level of patient compliance and public health protection.
Regional Insights
North America dominated the market in 2022. The region benefits from well-established healthcare infrastructure, robust research and development capabilities, and high healthcare expenditure, allowing for substantial investments in innovative vaccination technologies like microneedles. Additionally, the presence of leading pharmaceutical companies and research institutions fosters product development and clinical trials. On the other hand, Asia Pacific is expected to witness lucrative growth over the forecast period.
Key Companies & Market Share Insights
Key players operating in the market are Becton, Dickinson and Company, FluGen, Inc, Debiotech S.A, CosMED Pharmaceuticals Co., Ltd, NanoPass Technologies Limited,Microdermics, TSRL Inc. and Vaxess Technologies. In November 2022, Micron Biomedical, Inc. successfully secured USD14 million in Series A funding, which was expected to be directed towards bolstering the company's commercial manufacturing endeavors and forging a robust partnership between LTS Lohmann and Micron.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





