- Home
- »
- Medical Devices
- »
-
Middle East Cell And Gene Therapy CDMO Market, 2033GVR Report cover
![Middle East Cell And Gene Therapy CDMO Market Size, Share & Trends Report]()
Middle East Cell And Gene Therapy CDMO Market (2025 - 2033) Size, Share & Trends Analysis Report By Phase (Pre-clinical, Clinical), By Product (Gene Therapy, Gene-Modified Cell Therapy, Cell Therapy), By Indication (Oncology, Infectious Diseases), By Country, And Segment Forecasts
- Report ID: GVR-4-68040-760-4
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2021 - 2024
- Forecast Period: 2025 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Middle East Cell And Gene Therapy CDMO Market Summary
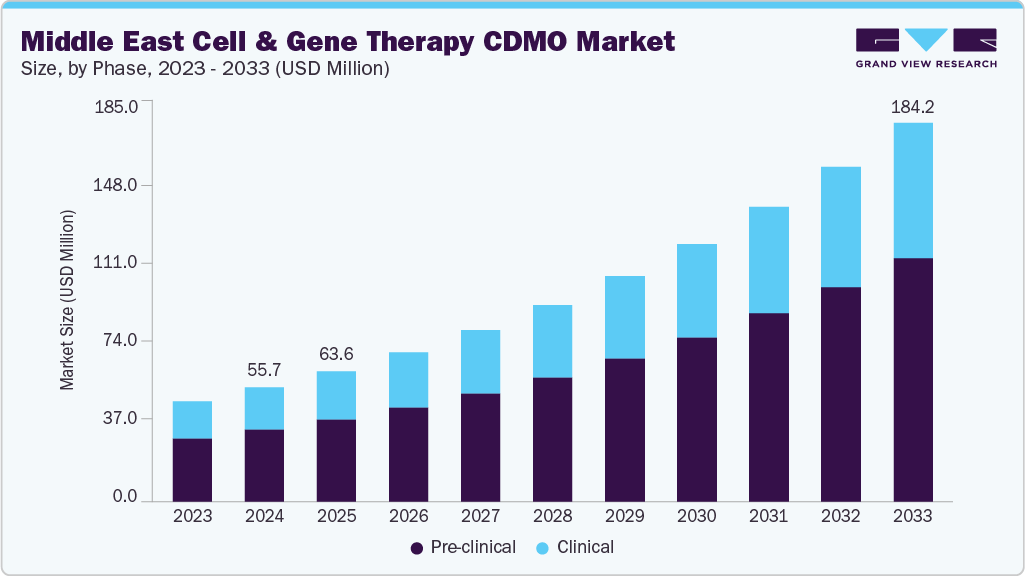
The Middle East cell and gene therapy CDMO market size was estimated at USD 55.68 million in 2024 and is projected to reach USD 184.18 million by 2033, growing at a CAGR of 14.23% from 2025 to 2033. The market is driven due to several factors, including rising investments in advanced therapeutics, increasing prevalence of genetic disorders, increasing government support for biotech innovation, and the growing need for specialized manufacturing services.
The market is primarily driven due to the region’s growing focus on biotechnology innovation and the rising demand for personalize and advanced therapeutic solutions. Governments across the region, mainly the UAE and Saudi Arabia, are actively investing in life sciences and precision medicine as part of their national development visions (Saudi Vision 2030). Thus, these factors have led to the creation of biotech hubs, innovation zones, and public-private partnerships, which are significantly boosting local capabilities in contract development and manufacturing. Moreover, the increasing cases of rare genetic disorders and cancers in the region are also one of the factors that are prompting both local and international biotech firms to outsource complex manufacturing processes to contract development and manufacturing organizations (CDMOs) specializing in gene-modified therapies, viral vector production, and cell engineering.
Furthermore, increasing strategic collaborations between regional healthcare systems and global pharmaceutical and biotech companies are also contributing to market growth. Several companies are tapping into the region's untapped market and are forming alliances with local CDMOs and research institutions to facilitate clinical development, scale-up production, and market entry. Moreover, regional regulatory authorities are making considerable initiatives to streamline approval processes for advanced therapies, establish centralized frameworks for clinical trials, and ensure GMP-compliant manufacturing environments. Thus, these factors are further fueling market growth.
Technological Advancements
Technological advancements are rapidly transforming the Middle East cell and gene therapy (CGT) CDMO landscape. It is enabling more efficient, scalable, and cost-effective manufacturing processes. Innovations such as closed-system automation, single-use bioreactors, and AI-driven process optimization are reducing contamination risks and production timelines, which is critical for personalized therapies like CAR-T. In addition, advances in viral vector production, gene editing tools (e.g., CRISPR), and next-generation sequencing further enhance product quality and regulatory compliance. These technologies present a compelling opportunity for Middle Eastern CDMOs to build state-of-the-art facilities that can attract global partnerships and contracts.
Phase Insights
Based on phase, the market is classified into pre-clinical and clinical segments. The pre-clinical segment held the largest revenue share of 63.5% in the Middle East market in 2024. The segment's growth is due to the increasing number of early-stage research collaborations, rising investments in proof-of-concept studies, and the region’s focus on developing local biotech capabilities. Governments and private investors across the UAE, Saudi Arabia, and Qatar are supporting early-stage innovation through grants and incubator programs, which have increased demand for pre-clinical development services, including process development, cell line engineering, and assay development.
The clinical segment is anticipated to grow at the fastest CAGR during the forecast period. The segment growth is driven by the increasing number of cell and gene therapy candidates progressing into clinical trials, rising regulatory support for advanced therapy medicinal products (ATMPs), and the expanding infrastructure for GMP-compliant manufacturing in the Middle East. Countries like the UAE and Saudi Arabia are investing significantly in biomanufacturing zones and clinical research centers, enabling regional CDMOs to support Phase I-III trials more effectively.
Product Insights
Based on product, the market is segregated into gene therapy, gene-modified cell therapy, and cell therapy segments. The cell therapy segment held the largest revenue share in the market in 2024. The segment's growth is due to the increasing adoption of stem cell-based treatments, a growing number of clinical trials for regenerative medicine, and supportive infrastructure for cell expansion and processing in the region. In addition, rising demand for autologous and allogeneic therapies to treat conditions such as cancers, autoimmune diseases, and degenerative disorders has contributed to the segment’s market growth.
The Gene-Modified cell therapy segment is anticipated to grow at the fastest CAGR during the forecast period. The segment growth is due to the increasing clinical success of CAR-T and TCR-T therapies, rising investments in personalized medicine, and technological advancements in gene editing tools like CRISPR and viral vector delivery systems. Moreover, strategic collaborations between regional CDMOs and international biotech firms are accelerating the development and localized manufacturing of these complex therapies, further driving the segment's rapid expansion.
Indication Insights
Based on indication, the market is segregated into oncology, infectious diseases, neurological disorders, rare diseases, and others. The oncology segment held the largest market share in 2024.due to the high prevalence of cancer across the Middle East, increasing demand for personalized cancer treatments, and the growing number of cell and gene therapy clinical trials targeting hematologic and solid tumors. Moreover, increasing approval rates of therapies such as CAR-T cells in treating refractory cancers has accelerated investment in oncology-focused CGT pipelines.
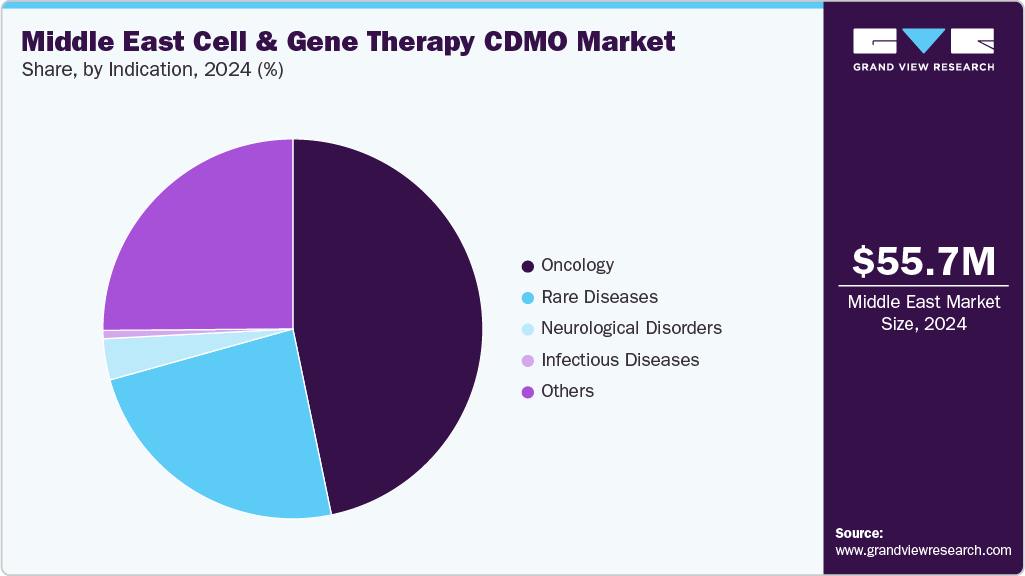
The rare diseases segment is anticipated to grow at the fastest CAGR during the forecast period. The segment growth is driven due to the increasing recognition of unmet medical needs in patients with orphan and ultra-orphan conditions. Regulatory incentives, such as fast-track designations and orphan drug statuses, are encouraging both local and international biopharmaceutical companies to focus R&D efforts on rare disease indications.
Country Insights
Saudi Arabia Cell And Gene Therapy CDMO Market Trends
The cell and gene therapy CDMO industry in Saudi Arabia held the largest market share in the Middle East region in 2024. The country’s growth is due to strong government support through initiatives like the National Biotechnology Strategy and Vision 2030, which aim to position the Kingdom as a regional biotech hub. Significant investments from the Public Investment Fund (PIF), including the launch of Lifera and the development of the country's first integrated ATMP manufacturing campus at King Faisal Specialist Hospital that are further contributing to the country's market growth.
UAE Cell And Gene Therapy CDMO Market Trends
The cell and gene therapy CDMO industry in the UAE held a significant share in 2024, owing to the country’s increasing clinical research activity, and a focus on localizing advanced therapy manufacturing. Key developments, such as the establishment of a CAR-T cell manufacturing facility at Burjeel Medical City in collaboration with Caring Cross, highlight the country’s commitment to reducing therapy costs and improving regional access to cutting-edge treatments.
Kuwait Cell And Gene Therapy CDMO Market Trends
The cell and gene therapy CDMO industry in Kuwait held a significant share in 2024. The growth of the market is due to increasing government interest in advanced therapies for prevalent genetic disorders like thalassemia and sickle cell disease. Early-stage research and pre-clinical development dominate the market, supported by expanding clinical services in local healthcare institutions such as the National Bank of Kuwait Hospital. The market is further boosted by rising demand for cell therapy raw materials and gene therapy starting materials, including viral vectors and plasmid DNA.
Key Middle East Cell And Gene Therapy CDMO Company Insights
The major players operating across the Middle East market are focused on adopting in-organic strategic initiatives such as mergers, partnerships, acquisitions, among others. Moreover, companies focus on technological innovations to augment their market position.
Key Middle East Cell And Gene Therapy CDMO Companies:
- Porton Pharma Solutions, Ltd. (Porton Advanced)
- WuXi AppTec
- Thermo Fisher Scientific, Inc.
- Charles River Laboratories
- Lonza
- AGC Biologics
- Catalent
- Samsung Biologics
- OmniaBio
- Rentschler Biopharma SE
Recent Developments
-
In July 2025, Porton Advanced and EVA Pharma signed an MoU to expand access to CAR T Cell-Therapy in the Middle East. This initiative aims to strengthen the CAR T Cell-Therapy development and manufacturing capabilities at EVA Pharma’s facilities.
-
In January 2025, Catalent entered a strategic collaboration with Galapagos NV to support decentralized manufacturing of GLPG5101, a CAR-T therapy for non-Hodgkin lymphoma. This partnership is designed to enhance patient access and accelerate clinical studies by leveraging local manufacturing sites.
Middle East Cell And Gene Therapy CDMO Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 63.55 million
Revenue forecast in 2033
USD 184.18 million
Growth rate
CAGR of 14.23% from 2025 to 2033
Actual data
2021 - 2024
Forecast period
2025 - 2033
Quantitative units
Revenue in USD million and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Phase, product, indication, country
Regional scope
MEA
Country scope
Saudi Arabia; Kuwait; UAE; Oman; Qatar
Key companies profiled
Porton Pharma Solutions, Ltd. (Porton Advanced); WuXi AppTec; Thermo Fisher Scientific, Inc.; Charles River Laboratories; Lonza; AGC Biologics; Catalent; Samsung Biologics; OmniaBio; Rentschler Biopharma SE
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Middle East Cell And Gene Therapy CDMO Market Report Segmentation
This report forecasts revenue growth at regional levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the Middle East Cell and Gene Therapy CDMO market report based on phase, product, indication, and country.
-
Phase Outlook (Revenue, USD Million, 2021 - 2033)
-
Pre-clinical
-
Clinical
-
-
Product Outlook (Revenue, USD Million, 2021 - 2033)
-
Gene Therapy
-
Ex-vivo
-
In-vivo
-
-
Gene-Modified Cell Therapy
-
CAR T-cell therapies
-
CAR-NK cell therapy
-
TCR-T cell therapy
-
-
Cell Therapy
-
-
Indication Outlook (Revenue, USD Million, 2021 - 2033)
-
Oncology
-
Infectious Diseases
-
Neurological disorders
-
Rare Diseases
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2021 - 2033)
-
Middle East
-
Saudi Arabia
-
UAE
-
Kuwait
-
Oman
-
Qatar
-
-
Frequently Asked Questions About This Report
b. The Middle East cell and gene therapy CDMO market size was estimated at USD 55.68 million in 2024 and is expected to reach USD 63.55 million in 2025.
b. The Middle East cell and gene therapy CDMO market is expected to grow at a compound annual growth rate of 14.23% from 2025 to 2033 to reach USD 184.18 billion by 2033.
b. Saudi Arabia dominated the Middle East cell and gene therapy CDMO market with a share of 39.03% in 2024. This is attributable to the country's strong government support through initiatives like the National Biotechnology Strategy and Vision 2030, which aim to position the country as a regional biotech hub.
b. Some key players operating in the Middle East cell and gene therapy CDMO market include Porton Pharma Solutions, Ltd. (Porton Advanced), WuXi AppTec, Thermo Fisher Scientific, Inc., Charles River Laboratories, Lonza, AGC Biologics, Catalent, Samsung Biologics, OmniaBio, Rentschler Biopharma SE
b. Key factors that are driving the market growth include rising investments in advanced therapeutics, increasing prevalence of genetic disorders, increasing government support for biotech innovation, and the growing need for specialized manufacturing services.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










