- Home
- »
- Medical Devices
- »
-
Neurothrombectomy Devices Market, Industry Report, 2030GVR Report cover
![Neurothrombectomy Devices Market Size, Share & Trends Report]()
Neurothrombectomy Devices Market (2025 - 2030) Size, Share & Trends Analysis Report By Product (Clot Retrievers, Aspiration/Suction Devices, Vascular Snares), By End Use (Hospital, Emergency Clinics, Ambulatory Surgical Centers), By Region, And Segment Forecast
- Report ID: GVR-2-68038-941-8
- Number of Report Pages: 154
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Neurothrombectomy Devices Market Trends
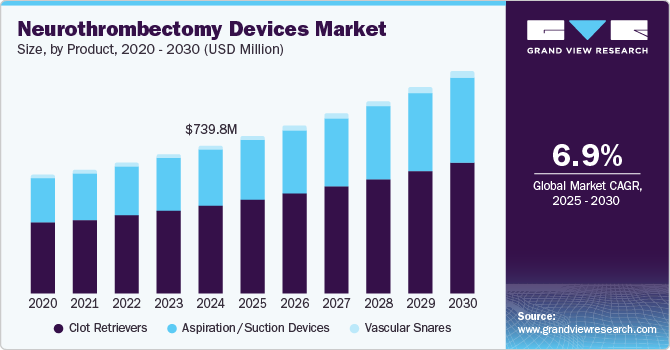
The global neurothrombectomy devices market size was estimated at USD 739.81 million in 2024 and is anticipated to grow at a CAGR of 6.94% from 2025 to 2030. Increasing incidences of acute ischemic stroke on a global level is majorly driving the market. Furthermore, increased adoption of unhealthy lifestyles and increasing awareness of the disorder among the population is fueling the market for neurothrombectomy devices. Neurothrombectomy devices are critical in treating ischemic stroke, a commonly occurring phenomenon of stroke among older adults. Modern lifestyle, stress, and dietary habits have been observed to result in the risk of stroke among adults older than 40 years.
Stroke is the leading cause of disability among the elderly, with around 65.0% of patients requiring physical assistance/support post an episode of stroke. Approximately 15 million people worldwide are expected to suffer from stroke each year, according to WHO. Since the neurothrombectomy procedure improves functional outcomes and lowers the mortality rate in patients, it is the most preferred treatment for acute ischemic stroke. Thereby boosting market growth over the forecast period.
Stroke is the most common cause of death, followed by heart and cancer diseases worldwide. For instance, according to WHO, in 2016, 15.2 million deaths of the 56.9 million deaths worldwide were due to stroke. Acute Ischemic Stroke (AIS) is the most common form of stroke, caused due to reduced blood supply to the brain, making brain cells die. For instance, according to the U.S. CDC, 87.0% of strokes are classified as ischemic strokes, with the U.S. being the most affected country According to Southwestern Medical Center, about 795,000 people in the U.S. suffer from a stroke every year. Furthermore, according to the National Center for Biotechnology Information (NCBI), strokes occur more commonly in women than men, especially in the elderly population (aged 55 to 65).
There are various initiatives undertaken by the government worldwide to prevent stroke. Some of the examples include the Well-Integrated Screening and Evaluation for Women Across the Nation (WISEWOMAN) program started by the U.S. CDC in three states-North Carolina, Massachusetts, and Arizona-to minimize the risk of stroke & heart diseases in women by promoting a heart-healthy lifestyle. These programs target uninsured women and low-income populations aged 40 to 64 in an attempt to spread awareness about heart diseases and other chronic conditions. From 2008 to 2013, nearly 91% of women who had strokes were offered effective care because of such awareness programs.
Moreover, the Global Stroke Bill of Rights campaign was started in 2014 by the World Stroke Organization for people suffering from stroke. This campaign helps patients avail effective care. In addition, with the help of Paul Coverdell National Acute Stroke Program, which started in 2005, CDC provides support and funding to healthcare departments of states to improve the quality of care for patients suffering from acute stroke disease. Since the initiation of this program, nearly 1 million stroke patients have been treated across 700 hospitals in the U.S. Thus, the abovementioned factors are expected to boost market growth.
Countries such as the U.S., UK, and Germany have witnessed a high incidence of hemorrhagic & ischemic stroke in recent years. According to the CDC, about 140,000 and 795,000 people in the U.S. die and suffer from stroke every year, respectively. In addition, the rising demand for minimally invasive procedures for treating neurovascular diseases further contributes to market growth in these countries. Similarly, the increase in mechanical thrombectomy procedures and the high adoption of technologically advanced products are major factors driving the market growth of German neurothrombectomy devices over the forecast period. For instance, as per an article published by BioMed Central Ltd in 2019, the rate of mechanical thrombectomy procedures has increased from 0.8% to 4.7% from 2010 to 2016. It was also reported that intravenous thrombolysis increased from 8.9% to 14.9% from 2010 to 2016. As intravenous thrombolysis and mechanical thrombectomy are major reasons for triggering acute ischemic stroke, increasing prevalence of these conditions is expected to boost the demand for neurothrombectomy devices, which is expected to drive the market.
Furthermore, the introduction of technologically advanced products and rapid product approval process are factors expected to fuel market growth over the forecast period. Various other technologies are used to develop neurothrombectomy devices for the treatment of AIS and its associated symptoms. For instance, in April 2019, Medtronic announced the launch of its fourth-generation Solitaire X revascularization device, designed for treating AIS. Furthermore, In May 2019, Vesalio received CE mark approval for NeVa-an advanced neurothrombectomy device featuring an optimized delivery system for efficient results. These devices expand on Drop Zone and Smart Maker technologies, introducing features such as distal filters.
Product Insights
The neurothrombectomy device market is segmented based on device type into aspiration/ suction devices, clot retrievers, and vascular snares. The clot retriever segment dominated the market for neurothrombectomy devices and accounted for the largest revenue share of 57.8% in 2024. Clot retriever devices are witnessing significant growth due to the rising prevalence of acute ischemic stroke and an increasing number of product launches by key market players. It is mainly used for removing blood clots from the cerebral arteries. For instance, in 2019, Medtronic announced the launch of Solitaire X revascularization device to remove blood clots in the brain. Furthermore, the use of clot retrievers has increased in elderly patients (aged 55 to 65) due to the growing incidence of stroke. Hence, growing cases of targeted disorder are propelling segment growth.
However, the aspiration/suction devices segment is expected to witness the highest CAGR of 7.3% from 2025 to 2030, owing to its increasing preference for physicians. In addition, these devices are witnessing growth due to a rise in the prevalence of neurological disorders among a large population. These devices vacuum clots out of the blood vessels and accurately re-vascularize large vessel occlusion. In addition, the growing use of the latest technology for treating stroke is a factor in increasing the overall growth of aspiration devices. In September 2018, Penumbra launched advanced Penumbra JET 7 and Penumbra JET D Reperfusion catheters featuring aspiration technology in the stroke thrombectomy segment. These devices are designed especially for physicians to enable extraction of thrombus safely through delivery of deep vacuum aspiration power of the penumbra engine. Thus, ongoing product launches are expected to increase the adoption of aspiration devices.
End Use Insights
Based on end use, the market is segmented into hospitals, ambulatory surgical centers, and emergency clinics. In 2024, the hospital segment dominated the market with a share of 70.4%. Hospitals are widely preferred by patients compared to emergency clinics due to improved healthcare facilities and the availability of advanced equipment. In addition, patients’ inclination towards the hospital since the patient gets treatment within eight hours of the onset of his symptoms is fueling segment growth.Increasing incidence of acute ischemic diseases and growing awareness about neurothrombectomy devices are factors expected to boost segment growth. Stroke is the leading cause of death and disability in the U.S. & Europe. Reperfusion therapy using neurothrombectomy devices is the preferred treatment for acute ischemic stroke. Hospitals remain the preferred choice for the treatment of acute ischemic disease than clinics as this disease is required to be treated within 8 hours of symptom onset. Thus, the increasing prevalence of stroke is driving segment growth.
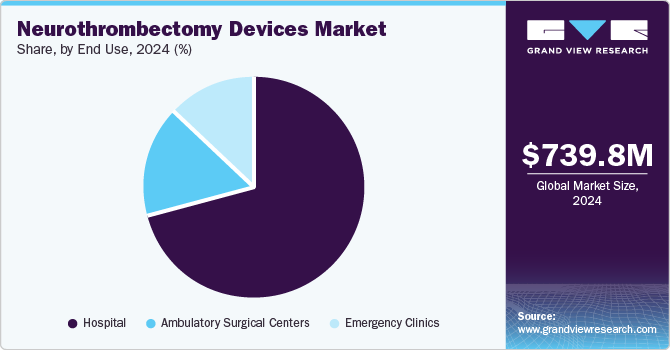
The ambulatory surgical centers (ASCs) segment is expected to grow at the highest CAGR of 7.6% during the forecast period. Since more than half of the outpatient surgeries are performed in ASCs, including acute ischemic disease surgical treatment, the treatment for this condition is required to be performed within 6 hours of symptom onset. In addition, a shorter waiting time offered by ASCs to patients is expected to drive the segment at a significant rate during the forecast period. Further, increasing demand for minimally invasive surgical procedures, introduction of technologically advanced products are expected to boost the neurothrombectomy devices market. For instance, in September 2018, Acandis GmbH announced CE mark approval for ACCERO Stent designed for use with embolization materials for treatment of intracranial aneurysms. It is the new self-expanding braided stent, featured by a smooth implant surface and superior wall apposition. Such advancements are expected to boost the segment growth over the forecast period.
Regional Insights
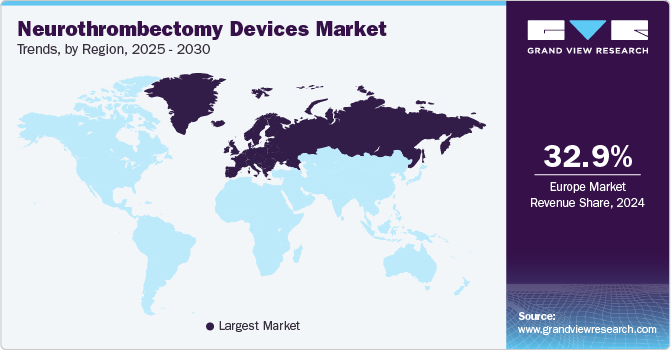
The North America neurothrombectomy devices market accounted for the highest share in 2024 and is expected to maintain this lead during the forecast period. Major factors contributing to the growth are the presence of key regional manufacturers, increased R&D investments, and a rise in government initiatives. For instance, in February 2024, the Leona M. and Harry B. Helmsley Charitable Trust and the American Heart Association revealed an investment of USD 5.05 million in South Dakota and USD 4.7 million in Minnesota under the Association’s Mission: Lifeline Stroke initiative to strengthen the full spectrum of stroke care across each state. Such initiatives undertaken by government authorities to improve stroke care are anticipated to propel regional market growth in the coming years.
U.S. Neurothrombectomy Devices Market Trends
Compared to other countries, the reimbursement policy in the U.S. neurothrombectomy devices market is favorable for providers and patients. The Centers for Medicare & Medicaid Services (CMS) is the regulatory body that oversees the reimbursement for medical devices and procedures in the U.S. CMS provides payment for inpatient stays through the Inpatient Prospective Payment System (IPPS), reimbursing acute care hospitals for each Medicare patient or case treated.
Canada Neurothrombectomy Devices Market Trends
The neurothrombectomy devices market in Canada is anticipated to be driven by the rising investment and funding for stroke care and the increasing incidence of stroke. The country's government is taking initiatives to improve access to stroke care. For instance, in September 2024, Heart & Stroke and Brain Canada, in partnership with the Government of Canada, invested USD 7.26 million to establish two new national research networks for brain and heart health.
Europe Neurothrombectomy Devices Market Trends
Europe neurothrombectomy devices market accounted for the largest revenue share of 32.9% in 2024. The region is expected to witness the highest CAGR of 7.5% from 2025 to 2030, owing to the increasing number of patients suffering from acute ischemic stroke. Europe is one of the most advanced regions globally, with innovative technologies and infrastructure that result in significant healthcare facilities and patient care. In addition, an increase in investments by government & private institutions is also expected to drive the market growth in this region. Moreover, increasing approvals for neurothrombectomy devices and the launch of new products by manufacturers in the region are expected to boost the market. Increasing government and NGO awareness initiatives worldwide are further adding to the segment's growth. For instance, in 2018, the Hospital Alliance for Europe (SAFE), with members from 12 countries and a non-profit organization, joined Boehringer’s Ineglheim’s (BI) Angel’s Initiative for providing critical information to stroke patients.
UK Neurothrombectomy Devices Market Trends
The UK neurothrombectomy devices market is anticipated to witness considerable growth over the forecast period due to the increasing burden of stroke in the country. According to the data published by the House of Commons, UK Parliament, more than 100,000 individuals suffer a stroke, and it is the fourth most significant cause of death in the UK.
Germany Neurothrombectomy Devices Market Trends
The Germany neurothrombectomy devices market is anticipated to witness significant growth in the coming years due to the rising adoption of mechanical thrombectomy procedures nationwide. For instance, A study published by the National Library of Medicine in January 2024 found that mechanical thrombectomy rates increased from 7.1% in 2019 to 8.4% in 2021, with the number of thrombectomy centers growing by 14.8% during the same period. This rise in procedure is expected to drive demand for neurothrombectomy devices in the coming years.
Asia Pacific Neurothrombectomy Devices Market Trends
The Asia Pacific neurothrombectomy devices market is majorly driven by the increasing incidence of acute ischemic stroke. In addition, emerging economies such as China, Japan, South Korea, and India are expected to grow considerably over the forecast period. Several local players in China and Japan may also boost the market growth.
Japan Neurothrombectomy Devices Market Trends
The growing geriatric population is one of the major driving factors for Japan neurothrombectomy devices market growth. According to the Al Jazeera Media Network, in September 2024, Japan's elderly population reached an all-time high of 36.25 million, with individuals aged 65 and over now making up nearly one-third of the country's total population, according to government data. The geriatric population is at a higher risk of various neurological diseases and disabilities. This factor may propel the demand for neurothrombectomy devices, leading to market growth.
China Neurothrombectomy Devices Market Trends
China neurothrombectomy devices market is expected to witness considerable growth over the forecast period owing to the increase in cases of acute ischemic stroke and the presence of several local & key players in the market. For instance, as per a report by NIH, by 2050, it is estimated that 4.07% of China's population will have experienced a stroke, representing a 101.49% increase compared to the 2.02% recorded in 2019.
Latin America Neurothrombectomy Devices Market Trends
The neurothrombectomy devices market in Latin America is anticipated to witness a moderate growth rate over the forecast period. Increasing awareness about treating acute ischemic stroke and increasing healthcare expenditure in Latin America are primary factors expected to drive the market over the forecast period. In addition, increasing healthcare expenditure in Brazil and the gradual adoption of advanced healthcare facilities may positively impact the market.
Brazil Neurothrombectomy Devices Market Trends
The Brazil neurothrombectomy devices market is anticipated to witness moderate growth over the forecast period. The increasing incidence of acute ischemic stroke is a major factor boosting the market growth. For instance, as per a report published by NCBI in 2022, in Brazil, acute ischemic stroke was the most prevalent form of stroke, with one-third of the population of Latin America suffering from stroke in Brazil.
Middle East & Africa Neurothrombectomy Devices Market Trends
The neurothrombectomy devices market in Middle East & Africa (MEA) region is anticipated to witness moderate growth over the forecast period. Middle Eastern countries such as Saudi Arabia, South Africa, and the United Arab Emirates (UAE) are some of the prospering economies. Increasing healthcare expenditure and gradually adopting technologically advanced products in these countries are expected to promote market growth.
Key Neurothrombectomy Devices Company Insights
The key players focus on adopting growth strategies, such as collaborations, partnerships, mergers, and new product launches. Market players are focused on adopting strategies such as marketing and promotions, and broadening product portfolios are widely used by companies to increase the availability and outreach of their product offerings.
Key Neurothrombectomy Devices Companies:
The following are the leading companies in the neurothrombectomy devices market. These companies collectively hold the largest market share and dictate industry trends.
- Medtronic
- Stryker Corporation
- Acandis GmbH
- Phenox GmbH
- Penumbra Inc.
- Vesalio
Recent Developments
-
In July 2024, Vesalio introduced the NeVa NET 4.0 mm, designed for treating acute ischemic stroke caused by large vessel occlusion. This launch comes from promising study results published in the Journal of Vascular and Interventional Neurology and NeuroInterventional Surgery.
-
In September 2024, MicroVention, Inc., a global leader in neurovascular innovation and a subsidiary of Terumo Corporation, officially rebranded as Terumo Neuro. This name change marks a significant milestone in the company's evolution, reaffirming its dedication to developing and delivering cutting-edge innovations in neurovascular care.
Neurothrombectomy Devices Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 787.98 million
Revenue forecast in 2030
USD 1.1 billion
Growth Rate
CAGR of 6.94% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Report updated
November 2024
Quantitative units
Revenue in USD Million and CAGR from 2025 to 2030
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Report coverage
Revenue, competitive landscape, growth factors, and trends
Key companies profiled
Medtronic; Stryker Corporation; Acandis GmbH; Stryker; Phenox GmbH; Penumbra Inc.; Vesalio
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Neurothrombectomy Devices Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global neurothrombectomy devices market report on the basis of product, end use and region:
-
Product Outlook (Revenue USD Million; 2018 - 2030)
-
Clot Retrievers
-
Aspiration/ Suction Devices
-
Vascular Snares
-
-
End Use Outlook (Revenue USD Million; 2018 - 2030)
-
Hospital
-
Emergency Clinics
-
Ambulatory Surgical Centers
-
-
Regional Outlook (Revenue, USD Million; 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
Colombia
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global neurothrombectomy devices market size was estimated at USD 739.81 million in 2024 and is expected to reach USD 787.98 million in 2025.
b. The global neurothrombectomy devices market is expected to grow at a compound annual growth rate of 6.9% from 2025 to 2030 to reach USD 1.10 billion by 2030.
b. Europe dominated the neurothrombectomy devices market with a share of 32.9% in 2024. This is attributable to the increasing number of patients suffering from acute ischemic stroke, increasing approvals for neurothrombectomy devices, and the launch of new products by manufacturers.
b. Some of the key players operating in the neurothrombectomy devices market include Medtronic, Stryker Corporation, Acandis GmbH, Stryker, Phenox GmbH, Penumbra Inc., Vesalio.
b. Key factors that are driving the neurothrombectomy devices market growth include the increasing prevalence of Acute Ischemic Stroke (AIS), rising adoption of an unhealthy lifestyle, and an increasing number of initiatives worldwide.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










