- Home
- »
- Medical Devices
- »
-
Pediatric Catheter Market Size, Share, Growth Report, 2030GVR Report cover
![Pediatric Catheter Market Size, Share & Trends Report]()
Pediatric Catheter Market (2023 - 2030) Size, Share & Trends Analysis Report By Product (Cardiovascular Catheters, Urology Catheters, Intravenous Catheters, Neurovascular Catheters, Specialty Catheters), By Region, And Segment Forecasts
- Report ID: GVR-3-68038-082-8
- Number of Report Pages: 82
- Format: PDF
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
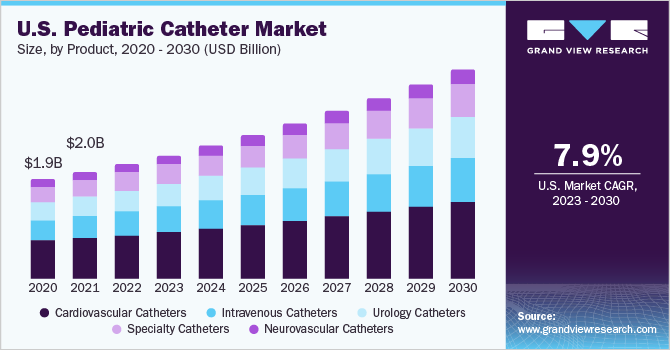
Pediatric Catheter Market Summary
The global pediatric catheter market size was estimated at USD 7.35 billion in 2022 and is projected to reach USD 14.97 billion by 2030, growing at a CAGR of 9.3% from 2023 to 2030. Growth in the pediatric population, rise in congenital heart defects (CHD), neurological disorders, urinary tract infections, and lack of adequate newborn screening centers in emerging countries are some of the factors promoting demand for pediatric catheter.
Key Market Trends & Insights
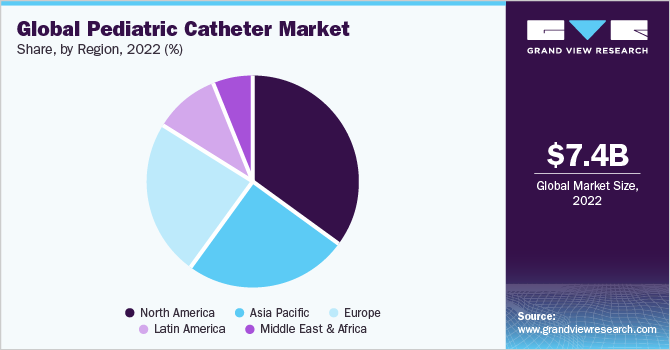
- The North America dominated the market with the largest revenue share of 34.4% in 2022.
- The Asia Pacific is expected to grow at the fastest CAGR of 11.2% over the forecast period from 2023 to 2030.
- Based on product, the cardiovascular catheters segment dominated the market with the largest revenue share of 39.9% in 2022.
Market Size & Forecast
- 2022 Market Size: USD 7.35 Billion
- 2030 Projected Market USD 14.97 Billion
- CAGR (2023-2030): 9.3%
- North America: Largest market in 2022
- Asia Pacific: Fastest growing market
Moreover, due to the increasing incidence of cardiovascular and urological diseases in the pediatric population, demand for minimally invasive surgeries is rising, which includes the use of catheters for surgical and diagnostic procedures. Also, technological advancements observed in pediatric catheters are anticipated to eliminate their associated infections in children and increase the demand for the use of catheters over the forecast period.
The demand for pediatric catheter devices experienced a significant boost as a result of the COVID-19 pandemic. According to the American College of Cardiology (ACC), around 40% of individuals who recovered from COVID-19 encountered cardiovascular complications. Therefore, there was a surge in the utilization of these devices, primarily driven by the rising need for intravascular ultrasound (IVUS) procedures during percutaneous coronary intervention (PCI) surgeries, as well as the growing acceptance of IVUS systems in medical diagnostic facilities and hospitals.
Product Insights
Based on product, the cardiovascular catheters segment dominated the market with the largest revenue share of 39.9% in 2022. Congenital heart defects pose a significant threat to the survival of pediatric patients, contributing to a high mortality rate within this population. One crucial factor that propels this trend is the availability of a diverse array of catheters designed for various interventional cardiology procedures. These catheters play a pivotal role in addressing the challenges posed by congenital heart defects, thereby influencing the segment's growth and impact on patient outcomes. Pediatric catheters are used to solve the issues that come with catheterization in youngsters. The design of pediatric catheter is unique, and it is frequently made of different materials. These help in the treatment of dysfunctional bladders caused by spinal cord damage, spina bifida, diabetes, stroke, Parkinson's disease, or incontinence. Due to the increased number of venous thromboembolism (VTE) in the pediatric population, the use of these devices has expanded globally. The ease of installation and inexpensive cost of these catheters are anticipated to drive the market demand.
Instances of alcohol and drug abuse during pregnancy are on the rise, raising the risk of congenital cardiovascular problems in newborns. Its prevalence is also linked to genetic predisposition. Urology gadgets are expected to be in high demand as more youngsters have urological diseases that necessitate rapid surgical intervention. The availability of numerous sizes for children of various ages, as well as a good ergonomic design, benefits in the post-surgery recovery process, and improved patient satisfaction, is driving the market growth.
The urology catheters segment is expected to grow at the fastest CAGR of 10.0% over the forecast period from 2023 to 2030. This high growth is anticipated on account of the increasing prevalence of bladder, urethra, uterus, and kidney problems in the pediatric population. Urology catheters are thin, hollow, plastic tubes used to drain urine from the bladder. The catheter is inserted into the bladder through the urethra. It is often used after operations for the urinary system as it allows the healing process. It aids in treatments related to the kidney, bladder, urethra, and reproductive organs. Discomfort related to the catheterization of the urinary tract is minimized by the use of the appropriate size of catheters in the pediatric population.
The use of catheters and their appropriate size varies with the age group of children. Thus, the increasing prevalence of urology diseases, such as urinary tract infections in infants, is driving the pediatric catheter market. Other products such as neurovascular, intravenous, and specialty catheters are also anticipated to expand at a high CAGR owing to the increasing incidence of corresponding diseases in children, which require the use of these catheters for diagnosis and treatment.
Regional Insights
North America dominated the market with the largest revenue share of 34.4% in 2022 owing to the growing prevalence of congenital heart defects, urological problems, and rising inpatient and emergency room procedures. Moreover, governments worldwide have implemented several initiatives with the objective of extending medical coverage to a large number of previously uninsured individuals. For instance, programs like the Child Health Insurance Program (CHIP) in the U.S. have been established to offer year-long healthcare coverage specifically to children. These initiatives are expected to have a positive impact on the adoption of pediatric catheter over the forecast period. Advanced healthcare infrastructure and favorable federal initiatives for the development of pediatric devices are likely to boost the market in North America. Rising incidence of congenital heart defects and urological problems in the region is also anticipated to emerge as a high-impact rendering factor in the market.
According to the estimates published by the U.S. Centers for Disease Control and Prevention (CDC), in 2018, one in four babies was born with heart defects and about 25% of newborns possess critical congenital heart defects that necessitate surgeries or other interventions to increase their chances of survival in the first year of their life. Moreover, the presence of advanced healthcare facilities, strong reimbursement scenarios, and favorable federal initiatives to encourage pediatric device development are anticipated to positively reinforce the growth in this region.
Asia Pacific is expected to grow at the fastest CAGR of 11.2% over the forecast period from 2023 to 2030, owing to the increasing prevalence rate of birth defects and expanding public and private healthcare infrastructure. According to estimates by the Asian Hospital and Healthcare Management, the prevalence rate of congenital heart defects (CHD) accounted for 9.3 per 1000 live births with a significantly high occurrence of pulmonary outflow obstructions and ventricular septal defects. The increasing prevalence of congenital heart defects and other anomalies in low and middle-income countries is fueling the growth of this market. Moreover, expanding public and private healthcare infrastructure is expected to boost revenue growth.
Key Companies & Market Share Insights
Market players implement various inorganic growth strategies, such as acquisitions, and organic growth strategies, such as the expansion of the product portfolio, to increase their market reach and presence. New product launches, along with technological advancements by market players, are expected to increase the competition in the market over the forecast period. Many of the major manufacturers are focusing on pediatric device research and innovation, which will help to expand their reach and attract a larger customer base. To strengthen their market positions, these suppliers use a variety of techniques, including product line expansion, acquisitions, and alliances. New product releases and technical innovations such as big analytics and robotics are expected to increase competition in the global market leading to maximizing opportunities in the market. For instance, in May 2022, Aesculap, Inc., the surgery division of B. Braun SE, announced its partnership with True Digital Surgery, a U.S. based company specializing in robotically controlled 3D digital visualization. Through this partnership, Aesculap, Inc. has successfully obtained long-term and exclusive distribution rights for the Aesculap Aeos digital surgical microscope, which has been jointly developed by the two companies. This partnership ensures the continued availability of the advanced Aesculap Aeos digital surgical microscope through authorized channels. The following are some of the major participants in the global pediatric catheter market:
-
BD
-
Coloplast Corp
-
Getinge
-
Boston Scientific Corporation
-
Johnson & Johnson Private Limited
-
ICU Medical, Inc.
-
Edwards Lifesciences Corporation
-
Medtronic
-
Cook
Pediatric Catheter Market Report Scope
Report Attribute
Details
The market size value in 2023
USD 8.01 billion
The revenue forecast in 2030
USD 14.97 billion
Growth rate
CAGR of 9.3% from 2018 to 2030
The base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
September 2023
Quantitative units
Revenue in USD million/billion, and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company share, competitive landscape, growth factors and trends
Segments covered
Product, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; Spain; France; Italy; Russia; Japan; China; India; South Korea; Singapore; Australia; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE
Key companies profiled
BD; Coloplast Corp; Getinge; Boston Scientific Corporation; Johnson & Johnson Private Limited; ICU Medical, Inc.; Edwards Lifesciences Corporation; Medtronic; Cook
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Pediatric Catheter Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global pediatric catheter market report based on product, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Cardiovascular Catheters
-
Urology Catheters
-
Intravenous Catheters
-
Neurovascular Catheters
-
Specialty Catheters
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Key factors driving the market growth include the growing prevalence of target diseases and rising demand for minimally invasive surgeries.
b. The global pediatric catheter market size was estimated at USD 7.35 billion in 2022 and is expected to reach USD 8.01 billion in 2023.
b. The global pediatric catheter market is expected to grow at a compound annual growth rate of 9.3% from 2023 to 2030 to reach USD 14.97 billion by 2030.
b. North America dominated the pediatric catheter market with a share of 34.4% in 2022. This is due to the growing prevalence of congenital heart defects and urological problems and rising inpatient and emergency room procedures.
b. Some of the key players operating in the pediatric catheters market include Cook Medical; Smiths Medical; Edwards Life Sciences Corporation; Medtronic Inc.; and Johnson & Johnson.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










