- Home
- »
- Medical Devices
- »
-
Pediatric Medical Devices Market Size & Share Report, 2030GVR Report cover
![Pediatric Medical Devices Market Size, Share & Trends Report]()
Pediatric Medical Devices Market Size, Share & Trends Analysis Report By Product (Cardiology Devices, In Vitro Diagnostic (IVD) Devices, Diagnostic Imaging Devices), By End-user, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-030-5
- Number of Report Pages: 110
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Report Overview
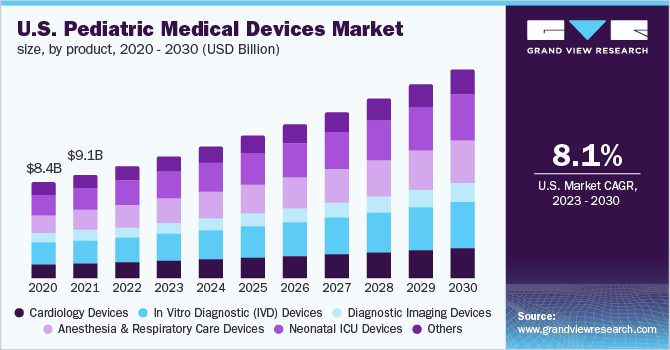
The global pediatric medical devices market size was estimated at USD 28.4 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 7.8% from 2023 to 2030. The increasing demand for pediatric medical devices due to the rising chronic diseases among the children such as respiratory disorders and asthma is expected to propel the growth of the market over the forecast period. The COVID-19 pandemic significantly impacted the pediatric medical devices market initially. Medical device companies have manufacturing facilities worldwide and some have been closed due to this pandemic. Transportation of medical devices is being delayed due to the lockdown, creating a supply shortage for many critical medical devices. The need for medical devices increased, and the restriction on the supply and delivery of medical devices was lifted.
The need for medical devices in hospitals, clinics, and other COVID-19 centers has fueled the demand and market growth. The FDA has initiated many actions to help ensure that patients have continued access to high-quality medical devices. It includes allotting Emergency Use Authorizations (EUAs) and other documents to provide approvals and help increase the availability and capability for various medical devices in high demand during the COVID-19 public health emergency.
Most of the drugs and devices in pediatric patients are used for off-label indications. As per the report published in the National Library of Medicine, around 60-75% of medical drugs or devices are used for off-label indications for pediatric patients. This results in drawbacks including performance and safety concerns with a lack of proper instructions and education for the use of an adult medical device for children.
The barriers to pediatric medical device manufacturing arise from the small numbers of pediatric patients due to the low volume of patients. There is a smaller number of clinical trial enrollments of children than in adult trials. Parental approval and consent also complicate the admission of children in the clinical trial process.
Despite legislative and regulatory changes that have encouraged the development of pediatric medical devices, only a small number of medical devices are submitted to the FDA every year for approval. Until 2018, only larger-sized valves were available and were used for heart surgery that is often not suitable for children's hearts. In 2018, the U.S. FDA has approved a rotatable mechanical heart valve of 15 mm size, the smallest device that allows surgeons to treat infants and newborns in need of aortic or mitral valve replacement.
Product Insights
The in vitro diagnostic (IVD) devices segment held the largest revenue share of 22.4% in 2022. Based on product, the market is segmented into cardiology devices, anesthesia, and respiratory care devices, in vitro diagnostic (IVD) devices, diagnostic imaging devices, neonatal ICU devices, and others. IVD devices conduct tests to detect disease, condition, or infection. Approximately 70% of all clinical decisions are made using IVD products due to their precision and accuracy.
The anesthesia and respiratory care devices segment is expected to exhibit the highest growth of 8.8% over the forecast period. The growth can be attributed to the fact of CAGR number of respiratory disorders among the children such as chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA). According to a report published in the Italian Journal of Pediatrics, respiratory diseases account for more than 25% of all pediatric consultations in which asthma accounts for 10% of the total cases.
End-user Insights
The hospitals segment held the largest revenue share of 40.6% as of 2022. The growing number of pediatric patient admissions and rising demand for advanced medical devices for better medical care are expected to propel the segment’s growth. According to a report published in the National Library of Medicine, more than 2 million children are admitted to hospitals in the U.S., incurring costs that represent 40% of pediatric healthcare expenditures.
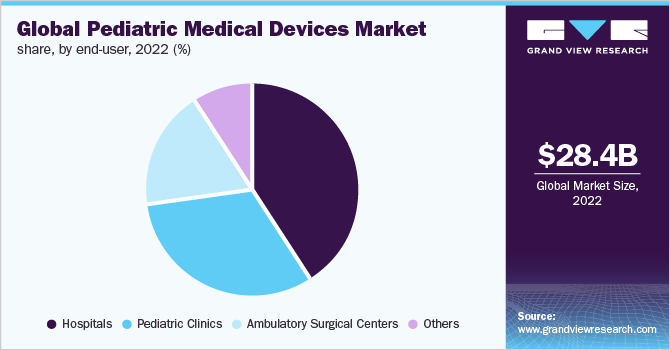
The pediatric clinics segment is anticipated to witness a lucrative CAGR of 8.4% over the forecast period. The growth can be attributed to the fact that these clinics are focused mainly on pediatric care. The pediatric clinics provide the latest clinical information on health issues for children and adolescents. It offers a full range of services to ensure that a child grows up healthily. These clinics also provide a full schedule of check-ups, vaccinations, and the overall development of a child.
Regional Insights
North America dominated the market in terms of a revenue share of 38.3% in 2022. The increasing rate of pediatric chronic diseases in the region such as asthma, cancer, congenital heart disease, leukemia, etc., and advanced medical devices are the factors propelling the growth of the market. As per the statistics of the Centers for Disease Control and Prevention, more than 40% of school-going children and adolescents have at least one chronic disease.
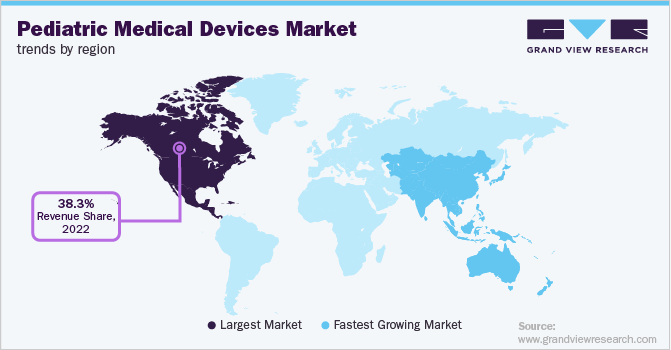
The Asia Pacific region is expected to showcase the highest CAGR of 8.7% over the forecast period. The growth can be attributed to the large population with a high prevalence of diseases among children such as kidney disease and birth defects, etc. The factors responsible for the growth of chronic diseases are the wealth status for affording healthcare services and the shortage of professional medical services in rural areas.
Key Companies & Market Share Insights
The market players are involved in the strategies such as product launches, strategic collaboration, and acquisition & mergers. The pediatric medical devices market is surrounded by many large and small players that contribute to the overall growth of the industry. Distribution channel improvement and collaboration with other industry players are some of the key initiatives undertaken by these market players.
For instance, in November 2022, GE Healthcare announced a $1 million donation of monitoring equipment and ultrasound to Ukraine as the war has a heavy toll on the country’s healthcare system. The company will be providing patient monitors devices, to monitor pediatric & adult patient vital signs. Some prominent players in the global pediatric medical devices market include:
-
TSE MEDICAL
-
Ningbo David Medical Device Co. Ltd
-
Hamilton Medical
-
GE Healthcare
-
Fritz Stephan GmbH
-
Phoenix Medical Systems Pvt Ltd
-
Novonate Inc.
-
Trimpeks
-
Atom Medical Corporation
-
Abbott
-
Medtronic PLC
Pediatric Medical Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 30.7 billion
Revenue forecast in 2030
USD 51.9 billion
Growth rate
CAGR of 7.8% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Market representation
Revenue in USD million & CAGR from 2023 to 2030
Report coverage
Revenue forecast, company share, competitive landscape, growth factors, and trends
Segments covered
Product, end-user, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country Scope
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Denmark; Sweden; Norway; China; India; Japan; South Korea; Australia; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
TSE MEDICAL; Ningbo David Medical Device Co. Ltd.; Hamilton Medical; GE Healthcare; Fritz Stephan GmbH; Phoenix Medical Systems Pvt Ltd.; Novonate Inc.; Trimpeks; Atom Medical Corporation; Abbott; Medtronic PLC
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional, and segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Pediatric Medical Devices Market Segmentation
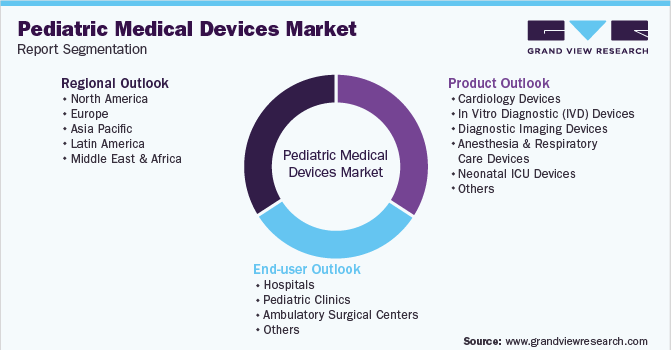
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global pediatric medical devices market report based on product, end-user, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Cardiology Devices
-
In Vitro Diagnostic (IVD) Devices
-
Diagnostic Imaging Devices
-
Anesthesia & Respiratory Care Devices
-
Neonatal ICU Devices
-
Others
-
-
End-user Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Pediatric Clinics
-
Ambulatory Surgical Centers
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
India
-
Japan
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Key factors that are driving the pediatric medical devices market growth include technological advancements in the medical devices industry and the growing need for advanced child care among the people.
b. The global pediatric medical devices market size was estimated at USD 28.4 billion in 2022 and is expected to reach USD 30.7 billion in 2023.
b. The global pediatric medical devices market is expected to grow at a compound annual growth rate of 7.8% from 2023 to 2030 to reach USD 51.9 billion by 2030.
b. North America dominated the pediatric medical devices market with a share of 38.3% in 2022. This is attributable to the fact of rising prevalence of chronic diseases among the children in the region.
b. Some key players operating in the pediatric medical devices market include TSE MEDICAL, Ningbo David Medical Device Co. Ltd., Hamilton Medical, GE Healthcare, Fritz Stephan GmbH, Phoenix Medical Systems Pvt Ltd., Novonate Inc., Trimpeks, Atom Medical Corporation, Abbott, Medtronic PLC.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





