- Home
- »
- Medical Devices
- »
-
Preterm Birth And PROM Testing Market Size Report, 2033GVR Report cover
![Preterm Birth And PROM Testing Market Size, Share & Trends Report]()
Preterm Birth And PROM Testing Market (2025 - 2033) Size, Share & Trends Analysis Report By Type, By Application (PROM, Preterm Labor, Chorioamnionitis), By Region, And Segment Forecasts
- Report ID: GVR-3-68038-055-2
- Number of Report Pages: 90
- Format: PDF
- Historical Range: 2021 - 2023
- Forecast Period: 2025 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Preterm Birth And PROM Testing Market Summary
The global preterm birth and PROM testing market size was estimated at USD 1.56 billion in 2024 and is projected to reach USD 3.26 billion by 2033, growing at a CAGR of 8.6% from 2025 to 2033. Increasing advancements in point-of-care diagnostics are expected to have a positive impact on the preterm birth and PROM testing market.
Key Market Trends & Insights
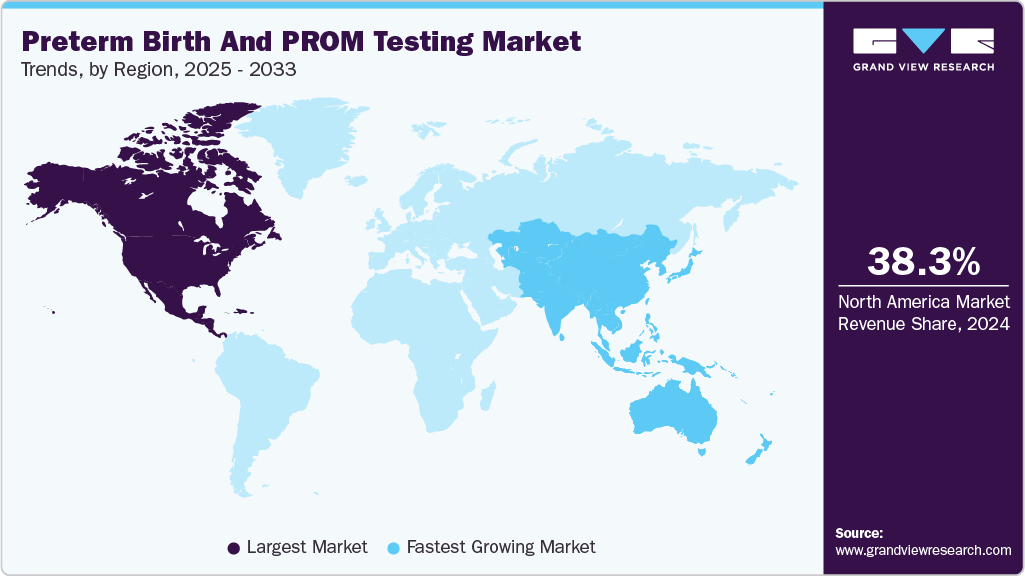
- North America preterm birth and PROM testing market held the largest share of 38.3% of the global market in 2024.
- The preterm birth and PROM Testing industry in the U.S. is expected to grow significantly over the forecast period.
- By type, the ultrasound segment held the highest market share of 31.5% in 2024.
- By application, the preterm labor segment held the highest market share in 2024.
Market Size & Forecast
- 2024 Market Size: USD 1.56 Billion
- 2033 Projected Market Size: USD 3.26 Billion
- CAGR (2025-2033): 8.6%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
The increasing average age of pregnant women, leading to an exponential rise in the number of early births, and the delay in parenthood due to sociodemographic factors, including education/career goals, the growing impact of women’s empowerment movements, and financial barriers, are key factors driving the adoption of tests in the market. According to research conducted by a group of researchers in Southern Ethiopia in December 2021, hypertension during pregnancy and a history of either abortion or cesarean section are the major causes of complications during childbirth. The study recommended that early identification tests and treatment can minimize the risk of premature rupture of fetal membranes. Other factors that raise the probability of PROM in pregnant women are low socioeconomic conditions, sexually transmitted infections, and cigarette smoking during pregnancy.According to UNICEF, India accounts for 25.0 million births each year and witnesses the death of one child every minute, with the mortality rate due to premature birth at 35.0%. While substantial development in Special Newborn Care Units (SNCUs) has led to a decline in neonatal mortality, the socio-cultural barriers in healthcare access for female infants remain a major factor behind higher female premature deaths. However, India has witnessed a consistent reduction in child mortality rates, driven by initiatives prioritizing newborn and maternal safety.

Governments and regulatory bodies are taking considerable measures to increase access to PROM testing across several regions. For instance, in March 2022, Sera Prognostics Inc. announced that the Centers for Medicare & Medicaid Services established a Medicare payment rate of USD 750 for its PreTRM test, a proteomic blood test designed to measure preterm birth risk. The test has gained additional traction, with ongoing commercial collaboration between Sera and Elevance Health regarding coverage for its PreTRM test, which improves affordability for high-risk pregnant women and encourages broader adoption among hospitals and maternal-fetal medicine specialists.
Similarly, Actim PROM is a U.S. FDA-approved POC rapid test, to detect PROM. It provides rapid accurate results for preterm births. More than 7 million tests have been distributed in over 70 countries since they are highly reliable with 97% sensitivity and specificity. Actim Prom helps detect the protein biomarker Insulin-like Growth Factor Binding Protein-1 (IGFBP - 1). The advancements in POC diagnostics for PROM can encourage pregnant women to test for PROM more frequently when compared to a long procedure of testing held in hospitals and clinics.
Type Insights
The ultrasound segment held the largest revenue share, of 31.5% of the global preterm birth and PROM testing market in 2024. Ultrasound imaging remains one of the most widely adopted modalities for assessing the risk of preterm childbirth, particularly through transvaginal measurement of cervical length. Recent market progression is shaped by digital innovation, as clinical researchers and companies increasingly integrate AI analytics with ultrasound platforms. In March 2022, Ultrasound AI Inc. received its first patent for Preterm AI, software that combines ultrasound data and machine learning to improve preterm birth prediction.
The PAMG-1 immunoassay segment is projected to register the fastest CAGR of 9.5% during the forecast period. Placental Alpha Microglobulin-1 (PAMG-1) is a highly specific biomarker used to confirm membrane rupture, where concentrations rise sharply (2,000-25,000 ng/mL) in the case of PROM or PPROM. Recent literature shows PAMG-1 may be useful as part of preterm-birth risk pathways (combined with cervical length, clinical risk) - creating new use cases beyond the classic PROM indication. That improves the addressable market.
Application Insights
The preterm labor segment held the largest revenue share of 42.4% in 2024. According to a Lancet publication (September 2021), China has the second-highest burden of preterm births, with a 1.3% annual increase in preterm rate from 2012 to 2018. Approximately 29.5% of preterm births originate from spontaneous preterm labor, while 27.8% are associated with preterm premature rupture of the fetal membranes (PPROM).
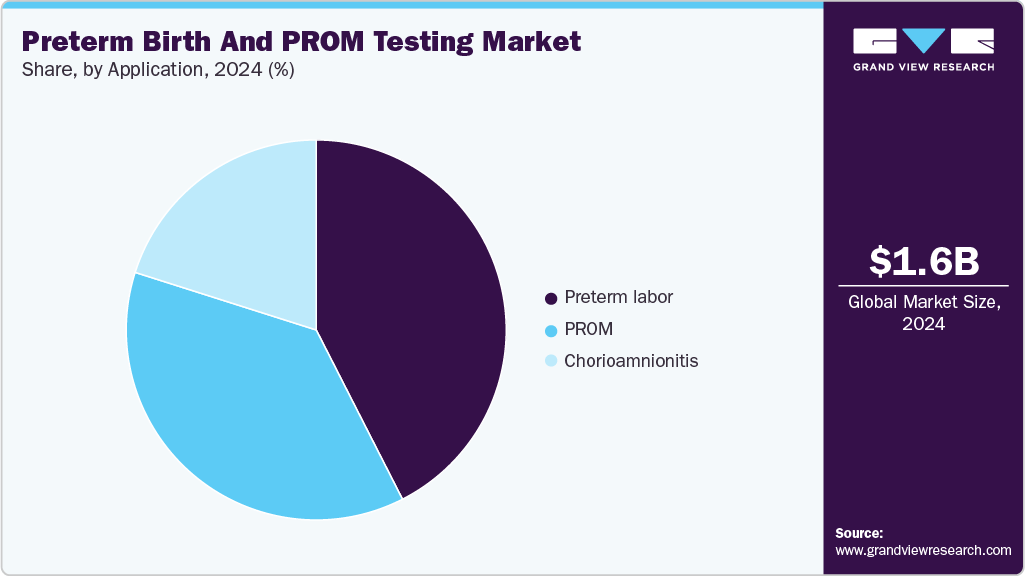
The Premature Rupture of Membranes (PROM) application segment is estimated to register a significant CAGR during the forecast period. According to data published in the NCBI in June 2021, the PROM condition occurs in 8% to 10% of pregnancies worldwide. For accurate and timely management of PROM, it is important to emphasize efficient diagnosis.
Regional Insights
North America preterm birth and PROM testing market held the largest revenue share of 38.3% in 2024 due to the changes in lifestyle factors such as excessive alcohol consumption & sedentary lifestyles, and high prevalence of preterm births in the region. The market is supported by strong institutional adoption of advanced prenatal diagnostics and well-established maternal healthcare systems.
U.S. Preterm Birth And PROM Testing Market Trends
The U.S. preterm birth and PROM testing market accounted for the largest share in North America in 2024, driven by a high clinical burden of preterm births and the rapid integration of innovative biomarker-based tests in routine obstetric care. Broader insurance coverage for diagnostic solutions, including risk-prediction assays and PROM POC kits, has improved patient access while supporting earlier clinical intervention.
Europe Preterm Birth And PROM Testing Market Trends
The Europe preterm birth and PROM testing market represented a significant market growth in 2024, supported by widespread use of established screening practices and stringent clinical management guidelines for high-risk pregnancies. Countries such as Germany, Italy, and the UK demonstrate strong uptake of PAMG-1 and IGFBP-1 biomarker testing as part of standardized care pathways.
Asia Pacific Preterm Birth And PROM Testing Market Trends
The Asia Pacific preterm birth and PROM testing market is expected to grow at the fastest CAGR of 9.1% over the forecast period, driven by the presence of a large population in India and China. Moreover, the rising number of preterm births in developing countries of the region is creating a significant potential market, thereby leading to high market growth. Rapid technological advancements and an increase in the number of awareness campaigns & nonprofit organizations supporting preterm infants and their families are some of the vital factors positively impacting the market.
The China preterm birth and PROM testing market held the largest revenue share in the Asia-Pacific region, driven by its high preterm birth burden, large patient population, and expanding network of advanced maternity hospitals. According to a Lancet publication (September 2021), China has the second-highest burden of preterm births, with a 1.3% annual increase in preterm rate from 2012 to 2018.
The India preterm birth and PROM testing market is emerging as a significant market within the Asia Pacific due to its high newborn population and ongoing efforts to reduce preventable preterm complications. Preterm births are one of the major causes of neonatal death in India. India enhanced high-risk pregnancy screening through programs such as LaQshya and SUMAN in 2023, expanding diagnostic access at district and community health levels.
Latin America Preterm Birth And PROM Testing Market Trends
Latin America presents steady growth opportunities driven by increasing utilization of point-of-care diagnostics in maternity emergency units and tertiary obstetric hospitals. Countries such as Brazil and Mexico are strengthening maternal-fetal health policies, encouraging the shift from traditional examinations toward more accurate biomarker testing for suspected membrane rupture.
Middle East & Africa Preterm Birth And PROM Testing Market Trends
MEA preterm birth and PROM testing market represents significant opportunities over the forecast period owing to a notable burden of risk factors such as maternal malnutrition, infectious diseases, and other socio-economic challenges in parts of Africa, which could increase preterm birth and PROM incidence. In addition, the rising awareness of preterm birth complications in selected African markets and GCC countries is pushing the demand for early detection and interventions.
Key Preterm Birth And PROM Testing Company Insights
The market is highly competitive with the presence of many international and local players. The major companies are focusing on strategies such as new product development, product enhancements, and regional expansion in an attempt to capture a large share of the market. For instance, in February 2023, Sera Prognostics announced positive results for its PreTRM Preterm Birth Test, a biomarker test based on blood used for predicting the risk of preterm birth. The individuals were managed with the test-and-treat approach, which resulted in a reduction of neonatal death and decreased length of hospital stay.
Key Preterm Birth And PROM Testing Companies:
The following are the leading companies in the preterm birth and PROM testing market. These companies collectively hold the largest market share and dictate industry trends.
- QIAGEN
- Hologic, Inc.
- CooperSurgical Inc.
- Abbott
- Medix Biochemica
- Sera
- Laborie
- Biosynex SA
- NX Prenatal Inc.
- IQ Products
Recent Developments
-
In May 2025, Mirvie launched Encompass, a predictive solution that uses a novel blood test analyzing cell-free RNA to identify personalized preeclampsia risk months before symptoms appear.
Preterm Birth And PROM Testing Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 1.68 billion
Revenue forecast in 2033
USD 3.26 billion
Growth rate
CAGR of 8.6% from 2025 to 2033
Base year for estimation
2024
Historical data
2021 - 2023
Forecast period
2025 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, trends
Segments covered
Type, application, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Russia; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Singapore; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key company profiled
QIAGEN; Hologic, Inc; CooperSurgical Inc.; Abbott; Medix Biochemica; Sera; Laborie; Biosynex SA; NX Prenatal Inc.; IQ Products
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Preterm Birth And PROM Testing Market Report Segmentation
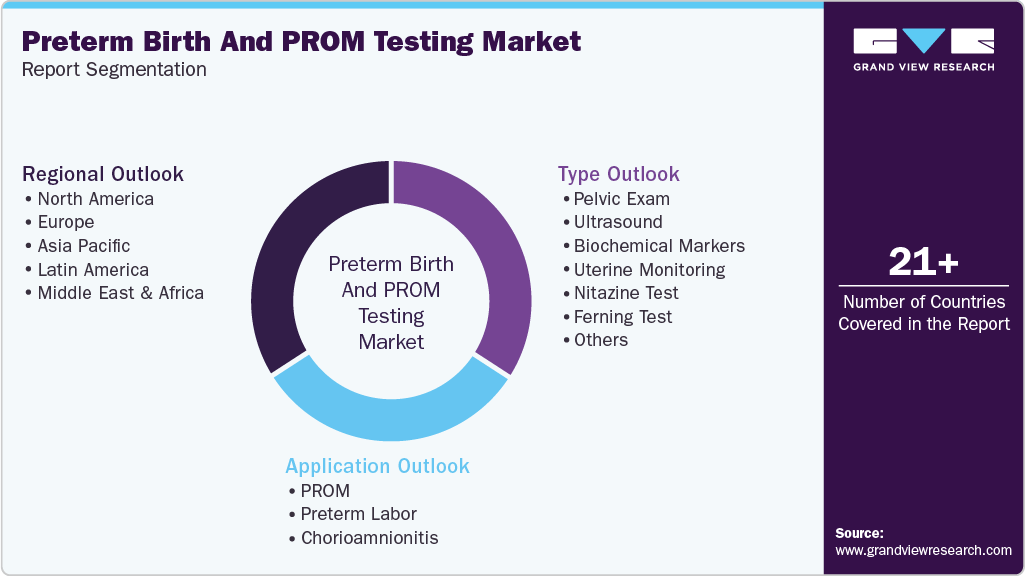
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the global preterm birth and PROM testing market report based on type, application, and region:
-
Type Outlook (Revenue, USD Million, 2021 - 2033)
-
Pelvic Exam
-
Ultrasound
-
Biochemical Markers
-
Interleukin (IL)-6
-
C-Reactive Protein (CRP)
-
IL-1, IL-2, IL-8, TNF-a
-
Corticotropin-Releasing Hormone (CRH)
-
AFP (Alpha fetoprotein)
-
-
Uterine Monitoring
-
Nitazine Test
-
Ferning Test
-
Pooling
-
PAMG-1 Immunoassay
-
IGFBP Test
-
Fetal Fibronectin (fFN)
-
Others
-
-
Application Outlook (Revenue, USD Million, 2021 - 2033)
-
PROM
-
Preterm Labor
-
Chorioamnionitis
-
-
Regional Outlook (Revenue, USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Russia
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Singapore
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. Some key players operating in the preterm birth and PROM testing market include Qiagen; Hologic; Cooper Surgical; Abbott Laboratories; Medixbiochemica; Sera Prognostics; Biosynex; and IQ Products.
b. Key factors that are driving the market growth include the growing incidence of preterm births, increasing geographical foothold of market players, high demand for effective preterm diagnosis, and improvement in point-of-care (POC) diagnostics.
b. The global preterm birth and PROM testing market size was estimated at USD 1.56 billion in 2024 and is expected to reach USD 1.68 billion in 2025.
b. The global preterm birth and PROM testing market is expected to grow at a compound annual growth rate of 8.6% from 2025 to 2033 to reach USD 3.26 billion by 2033.
b. North America dominated the preterm birth & PROM testing market with a share of 38.27% in 2024. This is attributable to the rising incidence of premature births, poor lifestyle choices, and increasing maternal age.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










