- Home
- »
- Pharmaceuticals
- »
-
Pseudomonas Aeruginosa Infection Treatment Market Report, 2030GVR Report cover
![Pseudomonas Aeruginosa Infection Treatment Market Size, Share & Trends Report]()
Pseudomonas Aeruginosa Infection Treatment Market Size, Share & Trends Analysis Report, By Treatment, By Route Of Administration, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-172-9
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Market Size & Trends
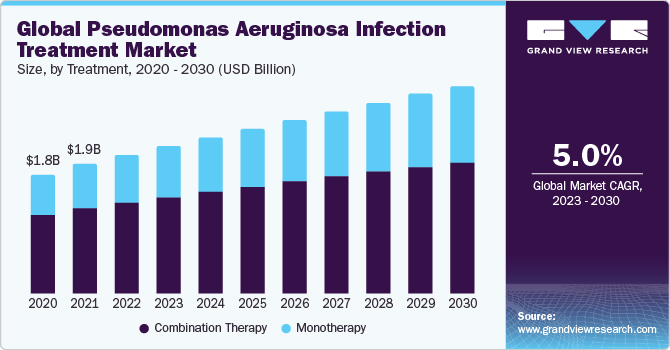
The global pseudomonas aeruginosa infection treatment market size was valued at USD 2.13 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 5.03% from 2023 to 2030. The pseudomonas aeruginosa infection treatment market is driven by the pathogen's high antibiotic resistance, necessitating innovative approaches like combination therapies. The emergence of extensively drug-resistant strains highlights the urgency for effective treatments, with a focus on overcoming antibiotic resistance challenges. The increasing incidence of infections further fuels the demand for advanced and targeted therapeutic solutions.
The pseudomonas aeruginosa infections have been on the rise, particularly in healthcare settings. This bacterium poses a significant threat, causing infections in vulnerable populations such as hospitalized patients, those with compromised immune systems, and individuals with chronic respiratory conditions. The prevalence of pseudomonas aeruginosa in healthcare-associated infections emphasizes the need for effective treatment strategies to address the growing burden of these infections.
Furthermore, the pseudomonas aeruginosa pathogen is widely recognized for its formidable resistance to commonly employed antibiotics, a trait stemming from both its inherent resistance mechanisms and its capacity to acquire additional resistance through genetic mutations. The inadequacy of conventional antibiotics in addressing infections caused by this pathogen poses substantial challenges. The rise of extensively drug-resistant strains, particularly evident in outbreaks, highlights the critical need for pioneering therapeutic strategies, notably combination therapies, to surmount the constraints imposed by antibiotic resistance.
Treatment Insights
On the basis of treatment, the pseudomonas aeruginosa infection treatment market is segmented into monotherapy and combination therapy. The combination therapy segment gained a maximum market share in 2022. This is due to the bacterium's notorious resistance to single antibiotics. Pseudomonas aeruginosa's ability to develop resistance necessitates a multifaceted approach, employing drugs with diverse mechanisms of action to enhance efficacy, prevent resistance, and improve patient outcomes.
This strategy, addressing the complexity of infections, aims to create a synergistic effect, making it more challenging for the bacteria to evade treatment. The combination therapy offers a broader spectrum of activity against various pseudomonas aeruginosa strains in severe or complicated cases, contributing to its prominence in the treatment landscape.
Route of Administration Insights
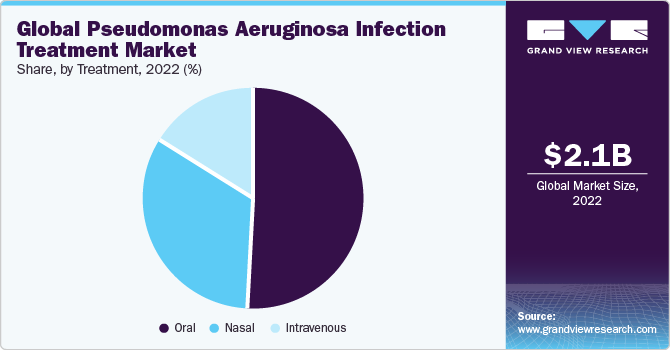
On the basis of the route of administration, the market is segmented into nasal, oral, and intravenous. The oral route of administration gained a maximum market share in 2022. This is due to its convenience and patient compliance. Oral medications offer a non-invasive and user-friendly method of treatment, making it easier for patients to adhere to prescribed regimens.
It is particularly crucial in the case of pseudomonas aeruginosa infections, where consistent and effective treatment is essential. Additionally, oral medications can be administered outside of healthcare settings, allowing for more flexible and accessible treatment options.
Regional Insights
North America dominated the market in 2022. In May 2023, the collaboration between the CDC, FDA, and state and local health departments actively addressed a nationwide outbreak of a drug-immune strain of Pseudomonas aeruginosa. This strain, known as Pseudomonas aeruginosa with integron-mediated metallo-β-lactamase Verona and extended-spectrum-β-lactamase Guiana (VIM-GES-CRPA), had not been documented in the U.S. before this outbreak. The outbreak spans various infection types, which include eye infections. This investigation serves as a significant driver for the pseudomonas aeruginosa Infection Treatment market. However, Asia Pacific is expected to grow at the fastest CAGR for the forecast period.
Key Companies & Market Share Insights
Key players operating in the market are AbbVie Inc. (Allergan PLC), Teva Pharmaceutical Industries Ltd, Pfizer Inc., Lupin Pharmaceuticals Inc., AstraZeneca PLC, Merck & Co. Inc., Bristol Myers Squibb Company, Johnson & Johnson, Baxter International Inc., Neopharma, CARB-X Company, and Sanofi SA. The market participants are constantly working towards new product development, M&A activities, and other strategic alliances to gain new market avenues. The following are some instances of such initiatives:
-
In September 2023, Orchid Pharma Ltd and the Global Antibiotic Research & Development Partnership (GARDP) entered into a sublicense agreement to produce cefiderocol, an antibiotic designed to address specific Gram-negative infections. This collaboration is a pivotal move within an ambitious initiative led by GARDP, Shionogi & Co. Ltd. (Shionogi), and the Clinton Health Access Initiative (CHAI), aiming to facilitate cefiderocol access in various primarily low- and middle-income countries, subject to local authorization or national regulatory approval.
-
In November 2022, Clarametyx Biosciences Inc. ("Clarametyx"), a biotech firm focused on the development of targeted immune-enhancing biologics for combating severe infections linked to biofilms, has officially commenced its clinical development initiative for CMTX-101. This innovative immune-enhancing antibody therapy is crafted to address critical bacterial infections. The technology employed facilitates the precise and swift eradication of the fundamental structure common to bacterial biofilms, disrupting bacterial defenses and enhancing the efficacy of antibiotic and immune interventions.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





