- Home
- »
- Clinical Diagnostics
- »
-
South East Asia COVID-19 Molecular Diagnostics Market Report, 2030GVR Report cover
![South East Asia COVID-19 Molecular Diagnostics Market Size, Share & Trends Report]()
South East Asia COVID-19 Molecular Diagnostics Market Size, Share & Trends Analysis Report By Product (Instruments, Reagents & Kits), By Technology (PCR), By End-user (Laboratories, Hospitals), By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-027-6
- Number of Report Pages: 180
- Format: PDF, Horizon Databook
- Historical Range: 2020 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Report Overview
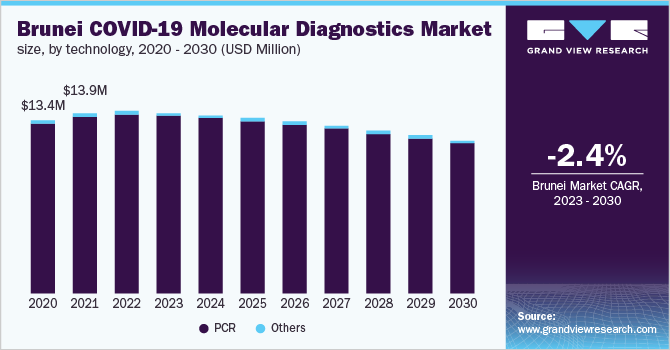
The South East Asia COVID-19 molecular diagnostics market size was valued at USD 2.27 billion in 2022 and is expected to decline at a compound annual growth rate (CAGR) of -5.20% from 2023 to 2030. Declining cases of COVID-19 in the region coupled with an increasing vaccinated population are some of the key factors restricting the market growth. Moreover, with the introduction of self-test kits, most people conduct tests themselves at home, which is also contributing to the declining number of RT-PCR tests performed.
The COVID-19 outbreak has increased the demand for diagnostic solutions. To cater to the growing need, a number of diagnostic companies are involved in robust R&D activities for the development of novel diagnostic tests, and they are also focused on obtaining approval from various regulatory bodies. For instance, in January 2022, Mammoth Biosciences received FDA Emergency Use Authorization (EUA) for its high-throughput CRISPR-based COVID-19 test, DETECTOR BOOST SARS-CoV-2 Reagent Kit. With this platform, it is possible to run thousands of tests per day with PCR-like performance.
Furthermore, constant government support has contributed to the South East Asia COVID-19 molecular diagnostics market. For instance, the Indonesian government implemented various policies to fight the coronavirus. The government launched the 3T program, which includes testing, tracking, and treatment to handle coronavirus. The aim was to make PCR test laboratories available in all the cities and districts, which could support regional hospitals.
However, rapidly spreading awareness about preventive measures for COVID-19 infections, implementation of strict physical distancing policies, and increased rate of vaccinations have played a prominent role in declining coronavirus cases in Southeast Asia. For instance, in October 2022, The Health Sciences Authority (HSA) announced authorizing the Pfizer bivalent COVID-19 booster vaccine in Singapore. These factors have restricted the market growth.
Product Insights
The reagents & kits segment held 78.4% of the overall market in 2022, as they were the key components of COVID-19 testing. The WHO urged regions to ramp up early detection of coronavirus, which has consequently driven the adoption of reagents & kits on a large scale. Regulatory bodies issued mass testing to combat the pandemic. Moreover, FDA provided emergency use authorization for several COVID-19 detection tests. This enabled key market participants to quickly obtain FDA approval for their testing reagents and kits.
The instruments segment contributed 21.6% of the total market revenue in 2022. The increased demand for diagnostic tests is proportionally accelerating the need for portable or on-site tests for efficient & accurate diagnosis. Such instruments are expected to help generate rapid and real-time patient management solutions. Moreover, this has increased the adoption of POC testing in COVID-19 diagnosis.
Technology Insights
The PCR segment dominated the South East Asia COVID-19 molecular diagnostics market in 2022, capturing a market share of 98.7%. This is because the majority of the detection tests use RT-PCR technology to measure the amount of RNA in samples from patients who have been exposed to the virus. The CDC, commercial labs, hospitals, and public health laboratories have all widely adopted PCR, which has contributed to the revenue generation of the segment.
In addition, several advanced technologies were also introduced for COVID-19 detection. These technologies include CRISPR-based diagnosis, amplicon-based metagenomic sequencing, and nucleic acid hybridization using microarray. While RT-PCR continues to be the most effective method for detecting SARS-CoV-2, it is essential to develop diagnostic assays that are quick, consistent, accurate, and reasonably priced.
End-User Insights
The laboratories segment accounted for the largest share of 42.8% in 2022. The pandemic created a significant need for new technologies to be deployed for diagnosing the infection. An increasing number of laboratories are using high throughput technologies to effectively conduct tests on a large scale. At the onset of the pandemic, several laboratories in South East Asia were working on conducting tests at a high scale. According to the data published by WHO, in June 2020, a COVID-19 testing facility was built in the Maldives, which could timely detect and roll out a response to COVID-19 cases. Approximately 700 samples could be tested per day, and the capacity would further be scaled up.
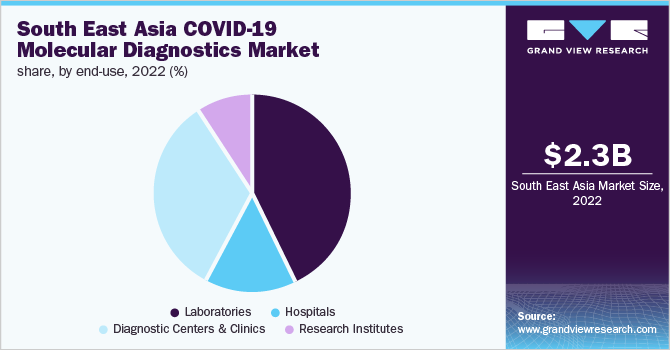
Diagnostic centers and clinics accounted for 33.2% of the revenue in 2022. Collaboration with several healthcare vendors and biotechnology institutes to increase testing capacity is one of the strategies implemented by clinics. Furthermore, initiatives by government agencies to provide cost-effective COVID-19 diagnostic services through laboratories have supported the segment’s growth.For instance, as part of the Australian government’s initiative for the Indo-Pacific region, the South East Asia laboratory Strengthening (SEALAB) project has been designed. The purpose is to strengthen laboratory systems in countries, such as Myanmar, Cambodia, and Laos.
Key Companies & Market Share Insights
Key companies operating in the market are focusing on strategic collaborations, partnerships, and geographical expansion, in economically and emerging favorable regions. For Instance, in November 2021, Thermo Fisher Scientific, Inc. confirmed that its PCR kits-TaqPath COVID-19 CE-IVD RT-PCR Kit and TaqPath COVID-19 Combo Kit-can detect the Omicron variant. This confirmation was expected to positively impact the company’s growth. Some prominent players in the South East Asia COVID-19 molecular diagnostics market include:
-
ThermoFisher Scientific, Inc.
-
F. Hoffmann-La Roche AG
-
Perkin Elmer, Inc.
-
Neuberg Diagnostics
-
1drop, Inc.
-
Veredus Laboratories Pte Ltd.
-
ADT Biotech
-
Altona Diagnostics GmbH
-
bioMérieux SA
-
Danaher Corporation
-
Mylab Discovery Solutions Pvt. Ltd.
-
Aldatu Biosciences
-
Quidel Corporation
-
Quest Diagnostics
-
Hologic, Inc.
-
Laboratory Corporation of America Holdings
-
Luminex Corporation
-
Abbott
South East Asia COVID-19 Molecular Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 2.23 billion
Revenue forecast in 2030
USD 1.54 billion
Growth rate
CAGR of -5.20% from 2023 to 2030
Base year for estimation
2022
Historical data
2020 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, technology,end-user, region
Country scope
Brunei; Cambodia; Indonesia; Laos; Malaysia; Myanmar; Philippines; Singapore; Thailand; Vietnam
Key companies profiled
ThermoFisher Scientific, Inc.; F. Hoffmann-La Roche AG; Perkin Elmer, Inc.; Neuberg Diagnostics; 1drop, Inc.; Veredus Laboratories Pte Ltd.; ADT Biotech; Altona Diagnostics GmbH; bioMérieux SA; Danaher Corporation; Mylab Discovery Solutions Pvt. Ltd.; Aldatu Biosciences; Quidel Corporation; Quest Diagnostics; Hologic, Inc.; Laboratory Corporation of America Holdings; Luminex Corporation; Abbott
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
South East Asia COVID-19 Molecular Diagnostics Market Segmentation
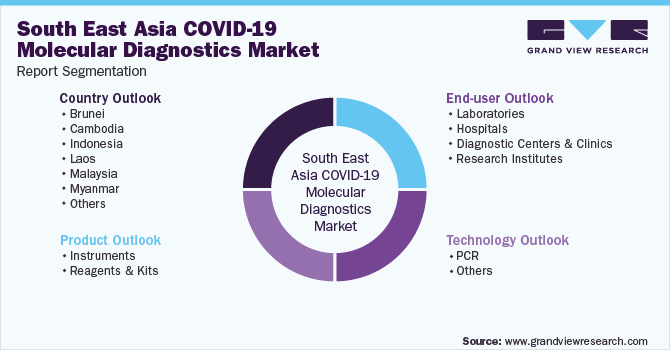
This report forecasts revenue growth at regional and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2020 to 2030. For this study, Grand View Research has segmented the South East Asia COVID-19 molecular diagnostics market report based on product, technology, end-use, and region:
-
Product Outlook (Revenue, USD Million, 2020 - 2030)
-
Instruments
-
Reagents & Kits
-
-
Technology Outlook (Revenue, USD Million, 2020 - 2030)
-
PCR
-
Others
-
-
End-user Outlook (Revenue, USD Million, 2020 - 2030)
-
Laboratories
-
Hospitals
-
Diagnostic Centers And Clinics
-
Research Institutes
-
-
Country Outlook (Revenue, USD Million, 2020 - 2030)
-
Brunei
-
Cambodia
-
Indonesia
-
Laos
-
Malaysia
-
Myanmar
-
Philippines
-
Singapore
-
Thailand
-
Vietnam
-
Frequently Asked Questions About This Report
b. The South East Asia COVID-19 molecular diagnostics market size was estimated at USD 2.27 billion in 2022 and is expected to reach USD 2.23 billion in 2023.
b. The South East Asia COVID-19 molecular diagnostics market is expected to witness a compound annual growth rate of -5.20% from 2023 to 2030 to reach USD 1.54 billion by 2030.
b. The Reagents & Kits segment accounted for the major revenue share as they were the key components of COVID-19 testing.
b. Major players in the South East Asia COVID-19 molecular diagnostics market include ThermoFisher Scientific, Inc., F. Hoffmann-La Roche AG, Perkin Elmer, Inc., Neuberg Diagnostics, 1drop, Inc., Veredus Laboratories Pte Ltd., ADT Biotech, Altona Diagnostics GmbH, bioMérieux SA, Danaher Corporation, Mylab Discovery Solutions Pvt. Ltd, Quidel Corporation, Quest Diagnostics, Hologic, Inc., Laboratory Corporation of America Holdings, Luminex Corporation, Abbott, and Aldatu Biosciences
b. Declining cases of COVID-19 in the Southeast Asian region coupled with an increasing vaccinated population are some of the key factors restricting the market growth
b. The PCR segment accounted for the major revenue share as Majority of the COVID-19 detection tests use RT-PCR technology to measure the amount of RNA in samples from patients who have been exposed to the virus.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





