- Home
- »
- Clinical Diagnostics
- »
-
Syndromic Multiplex Diagnostics Market Size Report, 2030GVR Report cover
![Syndromic Multiplex Diagnostics Market Size, Share & Trends Report]()
Syndromic Multiplex Diagnostics Market (2025 - 2030) Size, Share & Trends Analysis Report By Type (Respiratory, Gastrointestinal, Central Nervous System), By End Use (Hospitals, Diagnostic Laboratories, Others), By Region And Segment Forecasts
- Report ID: GVR-4-68040-507-5
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
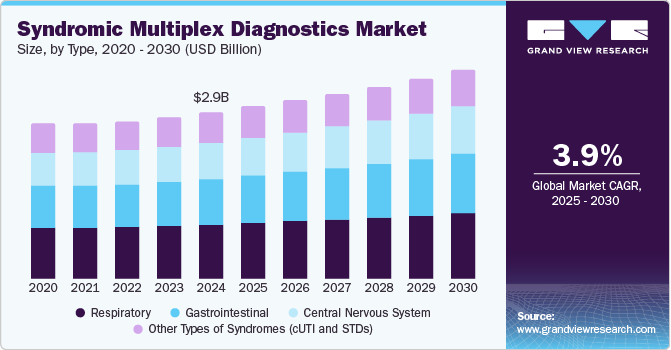
The global syndromic multiplex diagnostics market size was estimated at USD 2.92 billion in 2024 and is expected to expand at a CAGR of 3.91% from 2025 to 2030. The growth of the market is attributed to the rising demand for rapid, accurate diagnostics, advancements in diagnostics technologies, and rising prevalence of infectious diseases globally. Increasing incidences of infectious diseases such as respiratory infections, gastrointestinal diseases, and STIs among others have fueled the demand for syndromic testing. According to the World Health Organization (WHO), in 2023, tuberculosis (TB) caused 1.25 million deaths, including 161,000 among people with HIV. Moreover, an estimated 10.8 million people developed TB, including 6.0 million men, 3.6 million women, and 1.3 million children, affecting all countries and age groups. Such rise in infectious diseases increases the demand for effective diagnostics solutions thereby driving market growth.
Advancements in molecular diagnostics have also significantly driven the growth of the market. Next-generation sequencing and advanced Polymerase Chain Reaction (PCR) technologies have enhanced the sensitivity and specificity of multiplex assays, making them highly reliable. For instance, in September 2024, Roche launched the Cobas Respiratory Flex test, utilizing novel TAGS technology for syndromic multiplex PCR testing. This test can identify up to 15 respiratory pathogens from a single sample, including influenza and SARS-CoV-2. It enhances diagnostic efficiency by providing rapid results without needing additional samples, thereby streamlining laboratory processes and improving patient care. Such advancements significantly improve patient outcomes and drive the adoption of these diagnostics solutions in different healthcare settings.
Furthermore, increasing investments in research and development by market players are driving innovation, leading to the introduction of assays capable of detecting emerging pathogens and mutations, addressing critical unmet needs in healthcare. For instance, in September 2024, Korea’s Seegene partnered with Springer Nature to offer research grants aimed at advancing syndromic PCR diagnostic assays. The initiative seeks to enhance innovation in molecular diagnostics, encouraging researchers to develop new methodologies and applications. This collaboration indicates the importance of enhancing diagnostic capabilities for emerging infectious diseases, thereby contributing significantly to public health advancements. Such increasing investments and focus on R&D can lead to the development of innovative diagnostics solutions and drives market growth over the forecast period.
Market Concentration & Characteristics
The market is characterized by a high degree of innovation, driven by the rising prevalence of various infectious diseases, which increases the demand for effective diagnostics solutions. Manufacturers are introducing advanced solutions to address this increasing demand. For instance, in April 2022, Bruker introduced its innovative LiquidArray Multiplex PCR Assays, designed for high-throughput and simultaneous detection of multiple pathogens. This technology enhances diagnostic capabilities in clinical laboratories by allowing the analysis of various targets from a single sample. The assays are particularly beneficial for infectious disease testing, providing rapid results and improving patient management through efficient pathogen identification. Such advancements aim to streamline workflows and enhance diagnostic accuracy in healthcare settings.
The market is witnessing a significant level of mergers and acquisitions, and companies are adopting this strategy to increase their product portfolios and capabilities. For instance, in March 2021, Roche signed a definitive merger agreement to acquire GenMark Diagnostics, aiming to enhance its capabilities in syndromic multiplex testing. This technology allows for the simultaneous detection of multiple pathogens from a single patient sample, improving diagnostic efficiency and patient outcomes. The acquisition was aimed at strengthening Roche’s diagnostics capabilities.
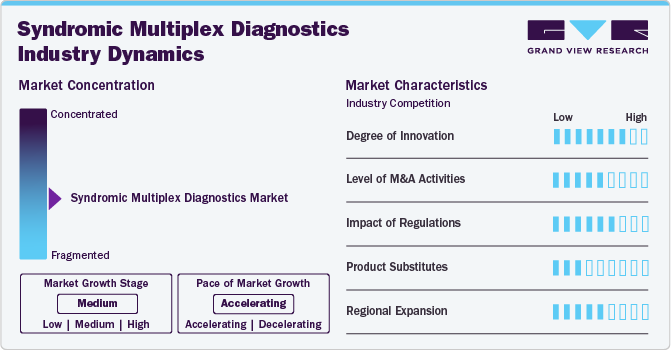
Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) play a crucial role in shaping the syndromic multiplex diagnostics industry. Their stringent guidelines ensure that diagnostic tests are safe, effective, and reliable. Compliance with these regulations can lead to increased costs and longer development times for manufacturers, potentially limiting innovation. However, regulatory approval also enhances market credibility and facilitates wider adoption among healthcare providers.
Traditional single-pathogen tests and newer point-of-care testing methods can serve as substitutes. However, these alternatives can lack the speed and efficiency of multiplex diagnostics, which can simultaneously detect multiple pathogens. Additionally, emerging technologies such as point-of-care testing and rapid antigen tests may offer convenience but usually do not match the comprehensive analysis provided by multiplex systems.
The syndromic multiplex diagnostics industry is witnessing significant regional expansion, particularly in emerging regions such as Asia-Pacific, Latin America, the Middle East, and Africa. For instance, in February 2024, Qiagen launched its QIAstat-Dx syndromic tests in India, designed for rapid and accurate diagnosis of infectious diseases. These tests utilize advanced technology to provide results within hours, enabling timely treatment decisions. The QIAstat-Dx platform can detect multiple pathogens simultaneously from a single sample, improving patient outcomes and streamlining laboratory workflows. This introduction aims to enhance diagnostic capabilities in India’s healthcare system, addressing the growing need for efficient infectious disease management.
Product Insights
The respiratory accounted for the largest revenue share of 32.70% in 2024 owing to the high prevalence and rapid spread of respiratory infections, coupled with the crucial need for accurate diagnosis. According to the WHO,seasonal influenza affects approximately one billion people worldwide each year, leading to 3-5 million cases of severe illness and resulting in 290,000 to 650,000 respiratory deaths annually. Moreover, the aging population, which is more vulnerable to respiratory illnesses, coupled with the increasing availability of effective respiratory diagnostics solutions further contributes to the segment growth.
The gastrointestinal segment is expected to witness significant growth owing to the increasing prevalence of gastrointestinal infections, requiring rapid and accurate diagnostic solutions. In addition to the rising incidence of GI infections, recent innovations in diagnostic technology are further propelling growth in this segment. For instance, in June 2024, QIAGEN launched the QIAstat-Dx Gastrointestinal Panel 2 in the U.S., following FDA clearance. This molecular test can identify up to 16 pathogens causing gastrointestinal infections within an hour, enhancing diagnostic accuracy and efficiency. It aims to reduce unnecessary treatments and improve patient care in gastrointestinal health. Such development of effective solutions is expected to drive segment growth over the forecast period.
End Use Insights
The diagnostics laboratories segment dominated the end use segments with the largest market share in 2024 due to their extensive infrastructure, high throughput capabilities, and crucial role in providing accurate, rapid, and comprehensive testing. Diagnostic laboratories also benefit from established partnerships with hospitals, clinics, and research institutions, increasing their need for effective diagnostics tools. Furthermore, the diagnostics expertise offered by these facilities significantly increases the patient footfall, further increasing demand for syndromic diagnostics tools in these facilities.
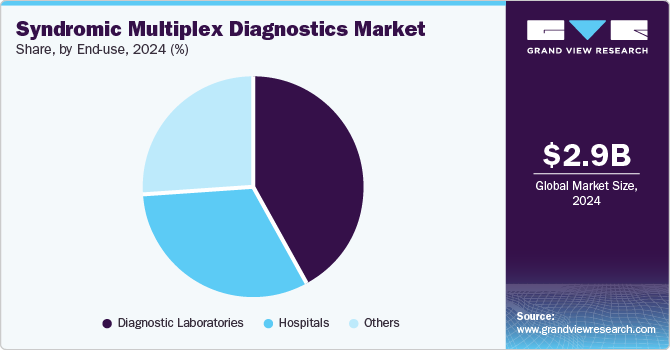
Hospitals are expected to witness significant growth in the syndromic multiplex diagnostics industry due to their high patient volume, advanced infrastructure, and need for rapid, accurate diagnostic solutions in acute care settings. Moreover, the increasing partnerships among hospitals and market players are increasing their adoption of diagnostics solutions. For instance, in August 2024, QIAGEN partnered with Parkway Laboratories to install its QIAstat-Dx diagnostic system in three of IHH Healthcare’s hospitals in Singapore, enhancing diagnostic accuracy for respiratory, gastrointestinal, and central nervous system infections. This system provides rapid, multiplex molecular testing, aiding in better patient management and antimicrobial stewardship. Such developments are further expected to fuel the segment growth over the forecast period.
Regional Insights
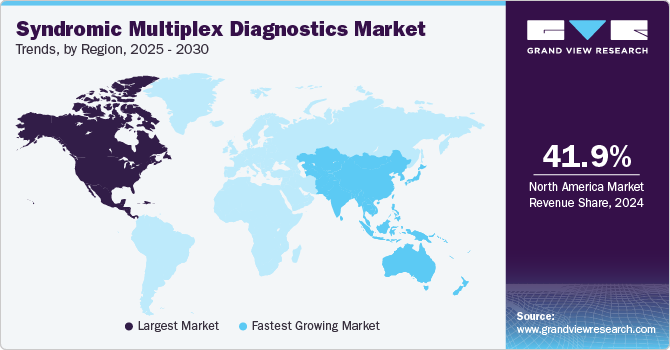
North America syndromic multiplex diagnostics market dominated the market and accounted for a 41.85% share in 2024. The syndromic multiplex diagnostics industry in North America is experiencing significant growth driven by the presence of key market players, evolving regulatory landscape, developed healthcare infrastructure, and increasing prevalence of various infectious diseases. For instance, according to the U.S. Department of Health & Human Services, approximately 1.2 million people in the U.S. are living with HIV, with 13% unaware of their status. In 2022, an estimated 31,800 individuals acquired HIV, and 37,981 people aged 13 and older were diagnosed with the virus across the U.S. and its territories. This high prevalence of infectious diseases significantly increases the demand for rapid diagnostics technology, which contributes to the market growth in the region.
U.S. Syndromic Multiplex Diagnostics Market Trends
The syndromic multiplex diagnostics market in the U.S. is expected to grow substantially over the forecast period. The major factors driving market growth include the high prevalence of various diseases, the aging population, technological advancements, and a supportive regulatory framework. Regulatory bodies, such as the FDA ensure the availability of effective diagnostics solutions in the country, which increases their credibility and adoption in the country’s healthcare sector. For instance, in May 2024, the FDA cleared Qiagen's QIAstat-Dx Respiratory Panel Plus, a syndromic test detecting 21 viral and bacterial pathogens causing respiratory infections. Utilizing real-time PCR, it delivers results in about one hour with minimal hands-on time. Syndromic testing enables co-infection detection, and enhances clinical decision-making, reducing healthcare burdens.
Europe Syndromic Multiplex Diagnostics Market Trends
The Europe syndromic multiplex diagnostics market is driven by the increasing prevalence of diseases, advancements in diagnostics technology, and the region's aging population. For instance, according to European Union statistics, in January 2023, the estimated population of the European Union was approximately 448.8 million, with over 21.3% of individuals aged 65 and older. The median age of the EU population was 44.5 years, indicating that half of the population was older than this age. This aging population is significantly vulnerable to various diseases, increasing demand for diagnostics technologies.
UK Syndromic Multiplex Diagnostics Market Trends
The syndromic multiplex diagnostics market in the UK is expected to grow over the forecast period due to the rising prevalence of various diseases, the country’s aging population, and evolving regulatory landscape. Moreover, the growing adoption of molecular diagnostics techniques further contributes to this growth.
Germany Syndromic Multiplex Diagnostics Market Trends
The syndromic multiplex diagnostics market in Germany is expected to grow significantly over the forecast period. Germany's comprehensive healthcare system, allowing rapid adoption of effective diagnostics solutions and increasing healthcare infrastructure are some of the major factors driving the market growth in the country.
Asia Pacific Syndromic Multiplex Diagnostics Market Trends
Asia Pacific syndromic multiplex diagnostics market is anticipated to witness the fastest growth over the forecast period. This can be attributed to the region’s developing healthcare infrastructure and the growing adoption of advanced diagnostics technologies. Many countries in the Asia-Pacific region, including China, Japan, and India, are experiencing a rapid demographic shift towards an aging population and a significant rise in various diseases. For instance, according to the Australian Institute of Health and Welfare, in 2021, over 362,600 hospitalizations occurred due to infectious diseases, with lower tract respiratory infections being the most common cause of hospitalizations.
China Pacific Syndromic Multiplex Diagnostics Market Trends
The syndromic multiplex diagnostics market in China is expected to grow over the forecast period owing to the increasing prevalence of infectious diseases, rising demand for rapid and accurate diagnostic solutions, advancements in healthcare infrastructure, and favorable government policies promoting diagnostic innovation. Additionally, healthcare facilities in the country are adopting advanced diagnostics technologies to address challenges such as delayed diagnoses and misdiagnoses, further contributing to market growth.
Japan Blood Syndromic Multiplex Diagnostics Market Trends
The syndromic multiplex diagnostics market in Japan is expected to grow over the forecast period due to the country’s aging population, the increasing prevalence of infectious diseases, advancements in molecular diagnostics, and supportive government initiatives promoting technological adoption in healthcare. According to the World Economic Forum, around 29.8% of the country’s population was aged above 65 years and above in 2021, making it the oldest population in the world. The older adults are more susceptible to infections, requiring precise and rapid diagnostic tools for effective treatment.
Latin America Syndromic Multiplex Diagnostics Market Trends
The syndromic multiplex diagnostics market in Latin America is witnessing substantial growth, driven by the high prevalence of infectious diseases, limited access to healthcare in rural areas, growing investments in healthcare infrastructure, and increased awareness about the benefits of advanced diagnostics. Many countries in the region are witnessing improvements in their healthcare systems, which contributes to market growth.
Brazil Syndromic Multiplex Diagnostics Market Trends
The syndromic multiplex diagnostics market in Brazil is driven by the high burden of infectious diseases, increasing investments in healthcare infrastructure, and government support for modern diagnostic technologies. Brazil faces ongoing challenges from diseases such as tuberculosis, and respiratory infections, which demand efficient diagnostic tools capable of identifying multiple pathogens simultaneously, thereby contributing to market growth.
Middle East & Africa (MEA) Syndromic Multiplex Diagnostics Market Trends
MEA syndromic multiplex diagnostics market is estimated to grow over the forecast period owing to the region's high burden of infectious diseases, limited access to timely diagnostics in underserved areas, rising investment in healthcare infrastructure, and increasing adoption of innovative medical technologies. Growing awareness among healthcare providers about the benefits of syndromic diagnostics, such as reduced diagnostic delays and improved treatment accuracy, is fueling market growth across the MEA region.
Saudi Arabia Syndromic Multiplex Diagnostics Market Trends
The syndromic multiplex diagnostics market in Saudi Arabia is growing rapidly, driven by a combination of factors, including the high burden of infectious diseases, growing government investment in healthcare modernization, increasing demand for advanced diagnostic tools, and the adoption of precision medicine. The Saudi government, as part of its Vision 2030 initiative, is heavily investing in the healthcare sector, focusing on the adoption of innovative technologies and the expansion of healthcare services to improve diagnostic accuracy and patient outcomes. Furthermore, Saudi Arabia’s emphasis on improving healthcare accessibility in rural areas has led to a growing adoption of point-of-care multiplex diagnostics.
Key Syndromic Multiplex Diagnostics Company Insights
Some of the key players operating in the market include Abbott Laboratories, Thermo Fisher Scientific, Inc., Abbott Laboratories, F. Hoffmann-La Roche Ltd and others. The market is highly competitive, with a large number of manufacturers accounting for a majority of the share. New source developments, mergers and acquisitions, and collaborations are some of the major strategies adopted by these players to counter the high competition.
Key Syndromic Multiplex Diagnostics Companies:
The following are the leading companies in the syndromic multiplex diagnostics market. These companies collectively hold the largest market share and dictate industry trends.
- Abbott Laboratories
- Thermo Fisher Scientific, Inc.
- F. Hoffmann-La Roche Ltd
- Qiagen
- bioMérieux
- DiaSorin S.p.A (Luminex Corporation)
- Hologic, Inc.
- Becton, Dickinson and Company (BD)
- Applied BioCode
- QIAGEN
- Curetis GmbH
Recent Developments
-
In December 2023, QuidelOrtho received FDA 510(k) clearance for its Savanna multiplex molecular platform and the Savanna HSV 1+2/VZV PCR assay, enabling rapid detection and differentiation of HSV-1, HSV-2, and VZV from patient lesion samples. The platform delivers results in approximately 25 minutes, enhancing diagnostic efficiency across various healthcare settings.
-
In November 2022, Cepheid launched the Xpert Xpress MVP, a new multiplexed PCR test, which can detect DNA from organisms associated with vulvovaginal candidiasis, bacterial vaginosis, and trichomoniasis from a single sample. This FDA-cleared test provides results within an hour, helping in more accurate diagnosis and treatment. =
-
In July 2021, QuantuMDx launched the Q-POC, a portable PCR diagnostic system capable of delivering rapid results in about 30 minutes. CE-IVD is marked for use in Europe, its initial SARS-CoV-2 assay shows high sensitivity and specificity. The device aims to address urgent diagnostic needs across various healthcare settings and plans to expand its testing capabilities.
Syndromic Multiplex Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 3.02 billion
Revenue forecast in 2030
USD 3.66 billion
Growth Rate
CAGR of 3.91% from 2025 to 2030
Actual data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD Million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, End Use, Region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Sweden; Denmark; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
Abbott Laboratories; Thermo Fisher Scientific, Inc.; F. Hoffmann-La Roche Ltd; Qiagen; bioMérieux; DiaSorin S.p.A (Luminex Corporation); Hologic, Inc.; Becton, Dickinson and Company (BD); Applied BioCode; QIAGEN
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Syndromic Multiplex Diagnostics Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global syndromic multiplex diagnostics market report on the basis of type, end use, region.
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Respiratory
-
Gastrointestinal
-
Central Nervous System
-
Other Types of Syndromes (cUTI and STDs)
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Diagnostic Laboratories
-
Others
-
-
Regional Outlook (Revenue in USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Denmark
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global syndromic multiplex diagnostics market size was estimated at USD 2.92 billion in 2024 and is expected to reach USD 3.02 billion in 2025.
b. The global syndromic multiplex diagnostics market is expected to grow at a compound annual growth rate of 3.91% from 2025 to 2030 to reach USD 3.66 billion by 2030.
b. North America dominated the syndromic multiplex diagnostics market with a share of 41.85% in 2024. This is attributable to the presence of key market players, evolving regulatory landscape, developed healthcare infrastructure, and increasing prevalence of various infectious diseases.
b. Some key players operating in the syndromic multiplex diagnostics market include Abbott Laboratories; Thermo Fisher Scientific, Inc.; F. Hoffmann-La Roche Ltd; Qiagen; bioMérieux; DiaSorin S.p.A (Luminex Corporation); Hologic, Inc.; Becton, Dickinson and Company (BD); Applied BioCode; QIAGEN
b. Key factors that are driving the market growth include the rising demand for rapid, and accurate diagnostics, advancements in diagnostics technologies, and rising prevalence of infectious diseases globally. Increasing incidences of infectious diseases such as respiratory infections, gastrointestinal diseases, and STIs among others have fueled the demand for syndromic testing.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










