- Home
- »
- Pharmaceuticals
- »
-
Vaccine Adjuvants Market Size, Share & Trends Report, 2030GVR Report cover
![Vaccine Adjuvants Market Size, Share & Trends Report]()
Vaccine Adjuvants Market Size, Share & Trends Analysis Report By Type (Pathogen, Adjuvant Emulsion, Particulate), By Administration (Oral, Intradermal, Intranasal, Intramuscular), By Application, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-612-7
- Number of Report Pages: 120
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Vaccine Adjuvants Market Size & Trends
The global vaccine adjuvants market size was valued at USD 1.62 billion in 2022 and is anticipated to grow at a CAGR of 2.3% from 2023 to 2030. The rising prevalence of cervical cancer, infectious diseases such as Human Papillomavirus (HPV), HIV, tuberculosis, etc., and other fatal diseases are expected to boost the demand for adjuvants. Besides, the growing focus on the long-lasting effect of immunization against existing and emerging diseases is expected to boost demand for adjuvants. The importance of adjuvant research for developing these products has increased significantly due to the inadequate immunogenicity of innovative vaccine antigens. Moreover, the increasing use of recombinant and synthetic vaccines is expected to accelerate the growth of this market in the coming years.
Major players are involved in discovering and developing new adjuvants to treat fatal diseases. Products under clinical trials include AS01, ISCOM & ISCOMMATRIX, and AS02, and the presence of these pipeline drugs is estimated to boost the market further.
The U.S. is a developed country with a huge population, and many travelers visit the country, which increases the need for immunization for the local population's safety. Also, a rise in favorable government initiatives for vaccinating people against serious diseases is anticipated to boost the market. For instance, the National Institute of Allergy and Infectious Diseases initiated a program for advancements in adjuvants for human use.
Application Insights
The infectious diseases segment accounted for the largest revenue share of 68.1% in 2022. This large share can be attributed to the gradually growing prevalence of infectious diseases, such as malaria, influenza, hepatitis A, B, & C, and others. Also, strong pipeline products for vaccine adjuvants related to infectious diseases are anticipated to fuel market growth in the coming years. This includes CpG (TLR 9 agonist) vaccine adjuvant from InvivoGen. The product is in phase 3 of the clinical trial and provides CD8 T cell-mediated and Th1-type immunity.
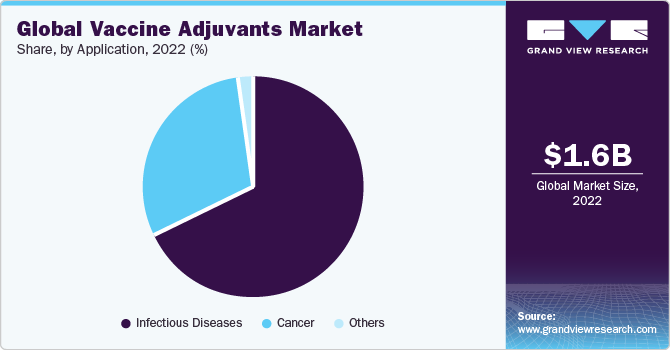
The other segment is expected to grow at the fastest CAGR during the forecast period. Cancer is identified to be the fastest-growing application segment due to its high prevalence and ongoing R&D for targeted therapy for various types of cancers. Moreover, increasing the use of adjuvants, such as Cervarix, is further anticipated to fuel growth.
Type Insights
The vaccine adjuvants market is segmented based on type into an adjuvant emulsion, pathogen, particulate, combination, and others. The particulate segment held the largest market share in 2022. This large share can be attributed to a wide range of products and greater efficiency against diseases.
Particulate type includes adjuvants consisting of alum, virosomes, and cytokines. Alum is one of the most used vaccine adjuvants. It consists of aluminum salts and has applications in vaccines such as HPV and hepatitis B. Virosome particles resemble some noninfectious viruses and are used as adjuvants in influenza, hepatitis A, and Epaxal vaccine.
The pathogen segment is expected to grow at the fastest CAGR over the forecast period. Monophosphoryl lipid A is one of the tested pathogens. It is combined with alum to produce AS04 adjuvant used in an HPV-Cervarix.
Regional Insights
North America dominated the market and accounted for the largest revenue share of 41.7% in 2022. The factors contributing to the growth of the market in the North American region are a rise in investments for R&D of new therapeutics and an increase in fatal epidemic diseases. Thus leading to the need for immunization against acute and chronic diseases. Also, the local presence of key market players in the U.S. increases the penetration of products. For instance, Adjuvance Technologies, Inc. has developed a portfolio of adjuvants to treat various diseases.
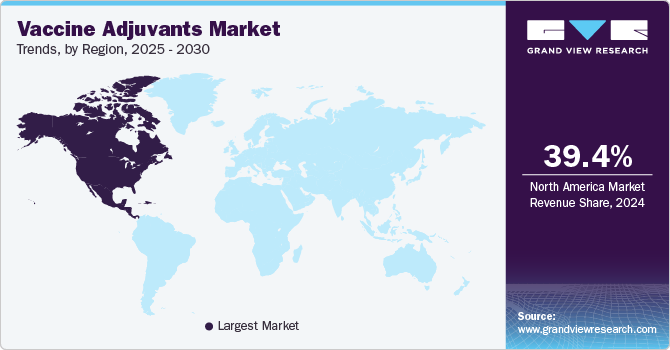
Middle East Africa is expected to grow at the fastest CAGR of 4.2% during the forecast period. Asia Pacific was estimated to be the fastest-growing market due to the continuously improving healthcare facilities, including the availability of medicines and rising R&D funding to meet the huge unmet demands. This is expected to increase the revenue share over the forecast period. For instance, in March 2022, Novavax and Serum Institute of India announced the first emergency authorization for using Novavax' COVID-19 Vaccine in Adolescents Aged 12 to 18 in India.
Administration Type Insights
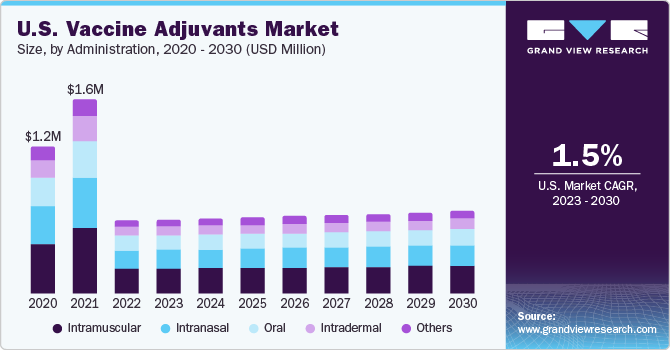
The administration segment is categorized into oral, intradermal, intranasal, intramuscular, and others. Due to better immune specificity, the intramuscular segment accounted for the largest revenue share of 33.8% in 2022. These include vaccines for HPV, influenza, meningitis, etc. The efficacy of vaccines depends on the route of administration. For instance, alum salts, the most widely used parenteral adjuvants, are ineffective when administered by the oral or nasal route.
On the other hand, the intradermal route is expected to be the fastest-growing segment due to longer absorption time and duration of action compared to other routes, increasing the demand for vaccines administered through this route. Some adjuvants, such as TLR3-Ligand Poly (I: C), induce mucosal antibody responses and protect against genital HSV-2 infection when administered intradermally.
Key Companies & Market Share Insights
The market players focus on expanding their businesses in developing regions to increase their market share. Furthermore, they are adopting collaborations, mergers & acquisitions, and new product development strategies. For instance, in July 2017, GSK collaborated with Exscientia to develop new drugs. In May 2023, GSK plc announced that the US Food and Drug Administration had authorized Arexvy to treat lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) for individuals age 60 and older. This is the world's first RSV vaccination for older individuals to be licensed. In March 2022, Croda International Plc announced the collaboration with Statens Serum Institute. They will manufacture and commercialize CAF, a range of patented cationic adjuvants developed by SSI.
Key Vaccine Adjuvants Companies:
- GlaxoSmithKline plc.
- Novavax, Inc.
- Adjuvance Technologies, Inc.
- SPI Pharma
- Agenus, Inc.
- CSL Limited
- InvivoGen
- Brenntag Biosector
Vaccine Adjuvants Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 1.7 billion
Revenue forecast in 2030
USD 1.9 billion
Growth Rate
CAGR of 2.3% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Report updated
November 2023
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, application, administration, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
GlaxoSmithKline plc.; Novavax, Inc.; Adjuvance Technologies, Inc.; SPI Pharma.; Agenus, Inc.; CSL Limited; InvivoGen; Brenntag Biosector
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
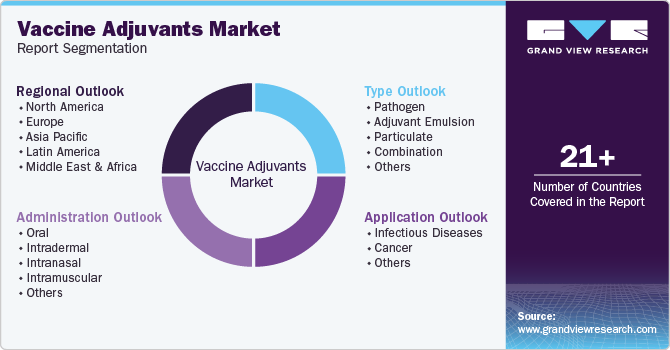
Global Vaccine Adjuvants Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global vaccine adjuvants market based on type, application, administration, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Pathogen
-
Adjuvant emulsion
-
Particulate
-
Combination
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Infectious diseases
-
Cancer
-
Others
-
-
Administration Outlook (Revenue, USD Million, 2018 - 2030)
-
Oral
-
Intradermal
-
Intranasal
-
Intramuscular
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global vaccine adjuvants market size was estimated at USD 1.62 billion in 2022 and is expected to reach USD 1.7 billion in 2023.
b. The global telemedicine market is expected to witness a compound annual growth rate of 2.3% from 2023 to 2030 to reach USD 1.9 billion by 2030.
b. North America held the largest share of 41.73% in 2022 due to the rise in investments for R&D of new therapeutics and an increase in fatal epidemic diseases. Moreover, the local presence of key market players in the U.S. increases the penetration of products booming the healthcare industry in the region.
b. Some of the key participants in the market are GlaxoSmithKline plc.; Novavax, Inc.; Adjuvance Technologies, Inc.; SPI Pharma.; Agenus, Inc.; CSL Limited; InvivoGen; and Brenntag Biosector.
b. The growing focus on the long-lasting effect of immunization against existing and emerging diseases is expected to boost the adoption of vaccine adjuvant over the forecast period.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





