- Home
- »
- Medical Devices
- »
-
Varicose Veins Treatment Devices Market Size Report, 2030GVR Report cover
![Varicose Veins Treatment Devices Market Size, Share & Trends Report]()
Varicose Veins Treatment Devices Market (2023 - 2030) Size, Share & Trends Analysis Report By Type (Endovenous Ablation, Sclerotherapy, Surgical Ligation & Stripping), By End Use, By Region, And Segment Forecasts
- Report ID: GVR-2-68038-177-1
- Number of Report Pages: 127
- Format: PDF
- Historical Range: 2017 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Report Overview
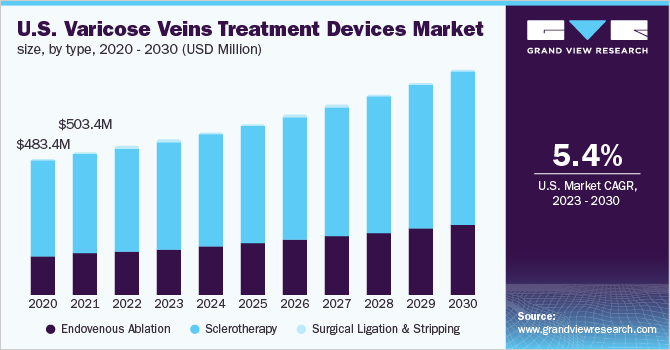
The global varicose veins treatment devices market size was valued at USD 1.3 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 6.32% from 2023 to 2030. Increasing demand for minimally invasive procedures to treat varicose veins, along with growing investments by the market players to develop innovative and effective products, is likely to boost the market growth. According to a study published by the Institute of Quality and Efficiency in Healthcare, Germany, it is expected that approximately 20% of all adults will develop varicose veins at some point during their life span. The elderly population is more prone to spider veins although they can also affect the young population.
Moreover, the increasing geriatric population is expected to foster the growth of the market. According to World Population Prospects 2019, the number of people aged 65 years and more accounted for 703 million and is expected to double, which is 1.5 billion by 2050 globally. Varicose veins are more common among older adults and women. For instance, according to an article published in the Journal of American Heart Association, approximately 23% of U.S. adults are estimated to have varicose veins. This is anticipated to favor market growth.
Varicose veins can be aesthetically displeasing. They become problematic only in case they cause pain, aching, swelling, and considerable discomfort. In severe cases, they may rupture and can lead to ulcers. Available options for the treatment of this condition have evolved over the last few years. The majority of private healthcare providers are offering improved minimally invasive techniques.
COVID-19 varicose veins treatment devices market impact: 2.4% decrease from 2019 to 2020
Pandemic Impact
Post COVID Outlook
The pandemic has had adverse impacts on the manufacturers as well as users. As varicose veins procedures are non-urgent, the volume of procedures declined drastically.
The demand for vascular veins treatment devices is growing due to a decline in the number of COVID patients and relaxations in restrictions. The sales are climbing for the vascular segments of the companies operating in the market. For instance, the endovascular therapies segment net sales of AngioDynamics increased by 72.3% in the fourth quarter of the year 2021.
Reduction in the surgeries or procedures for the treatment of varicose veins due to traveling restrictions also impacted the sales of the manufacturers. For instance, net sales of AngioDynamics decreased by 2.4% in 2020 compared to the previous year.
Mergers and acquisitions, new product launches, and technological advancements in the field of varicose vein treatment devices are expected to drive the market. For instance, in December 2021, Becton, Dickinson, and Company (BD) acquired Venclose Inc. for the expansion of its business in the treatment of Chronic Venous Insufficiency.
The elderly population is more prone to the development of varicose veins. The effect of aging on the structure and functions of the veins is found to be considerable. Over the age of 50, one out of every two people is affected by varicose veins. Overweight people and pregnant women are also susceptible to developing this condition. Some of the treatments available in the market include sclerotherapy, laser therapy, and radiofrequency ablation. Sclerotherapy is preferred as a first-line of treatment for varicose, spider, and reticular veins.
Varicose veins surgical procedures are likely to undergo significant changes. Surgery has been largely replaced by noninvasive options. Due to the risk of infections associated with invasive surgeries, preference for noninvasive surgeries has increased in recent years. This factor is projected to drive the market during the forecast period. In addition to the risk of infection, noninvasive methods also involve minimum hospital stays, thus saving time and cost. More surgeons are adopting sclerotherapy, endovenous laser treatment, and radiofrequency ablation over conventional surgical practices.
Type Insights
The sclerotherapy segment accounted for the largest revenue share of over 70.0% in 2022. Advantages of this treatment such as less bruising and scarring, no need for sedation, reduced hospital stay, simple follow-up treatments, and positive reimbursement policies are responsible for the dominant share of the segment. Moreover, in the U.S., Medicare provides coverage to patients undergoing sclerotherapy treatment. Based on type, the market is segmented into endovenous ablation, sclerotherapy, and surgical ligation and stripping.
Various organizations such as the British Association of Sclerotherapies (BAS) undertake initiatives annually for raising awareness and encouraging the adoption of various treatments. An increasing number of product approvals is also contributing to the market growth. For instance, in November 2020, Vascular Barcelona Devices (VB Devices) received approval of CE mark for Varixio Pod Air as Class 1 s medical device intended for automated preparation of foam for varicose veins sclerotherapy.
The endovenous ablation segment is likely to witness the fastest CAGR of 7.08% during the forecast period. The endovenous ablation procedure is preferred over the conventional procedures, such as surgical stripping and ligation, as the procedure is minimally invasive and is associated with lesser complications. Moreover, various market players are focusing on the development of efficient and technologically advanced products, which is likely to boost market growth.
Regional Insights
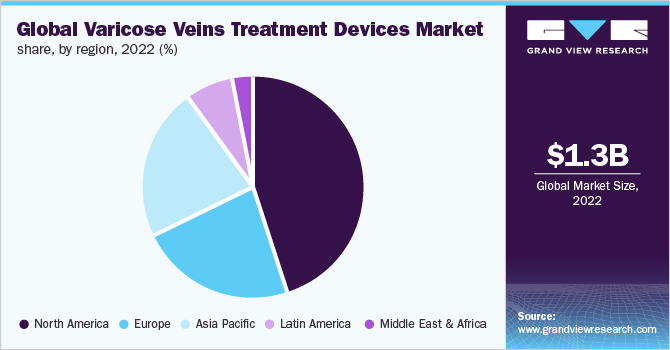
North America dominated the market with a revenue share of over 45.0% in 2022. This is mainly due to a large patient base, which is aesthetically conscious. People are willing to undergo cosmetic procedures owing to the evident results and safety. The demonstration of the safety and efficacy of laser treatments has helped reduce the stigma attached to cosmetic procedures and has helped gain social acceptance for laser procedures, especially among men.
In the U.S., the Society for Vascular Surgery has created clinical care guidelines along with the American Venous Forum for the treatment of varicose veins. These guidelines offer recommendations for the management of chronic venous diseases, based on the grading system. Grading the severity of the condition helps care and insurance providers communicate with one another. For instance, while referring patients to vein specialists, a general physician can effectively communicate the severity. Patients can be classified into different groups based on their severity.
Asia Pacific is expected to witness fastest growth with a CAGR of 7.71% during the forecast period. The Asia Pacific is expected to witness substantial growth in the forthcoming years due to the presence of a large elderly population, increasing disposable income, and rising awareness regarding these procedures. The market is highly diverse due to varying regulatory factors, which result in the difference in the availability of products in different countries.
Key Companies & Market Share Insights
Product launches, mergers and acquisitions, and investments in R&D for portfolio expansion are some of the key strategies undertaken by the major players for strengthening their market position. For instance, in August 2019, Surekha Varicose Veins Mumbai launched India’s first-ever Cryo Laser and Cryo Sclerotherapy (CLaCS) for the treatment of small varicose veins in the leg and spider veins. This technology consists of AR (Augmented Reality), Transdermal laser, and sclerotherapy.
The leading company in terms of revenue was Medtronic, followed by Teleflex Incorporated. The dominant position of these companies can be attributed to the broad product portfolio, higher penetration at the global level, and various strategic initiatives. For instance, in March 2021, Medtronic launched an Investigation Device Exemption (IDE) study to determine the effectiveness and safety of the Abre venous self-expanding stent system to be used in the treatment of deep venous diseases. Around 200 subjects are likely to be enrolled in this study across 35 locations throughout the U.S. and Europe. Some prominent players in the global varicose veins treatment devices market include:
-
AngioDynamics
-
Medtronic
-
Teleflex Incorporated
-
Sciton, Inc.
-
Dornier Medtech
-
Merit Medical Systems
-
Alma Lasers
-
Biolitec AG
-
Boston Scientific Corporation
Varicose Veins Treatment Devices Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 1.4 billion
Revenue forecast in 2030
USD 2.1 billion
Growth rate
CAGR of 6.32% from 2023 to 2030
Base year for estimation
2022
Historical data
2017 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Japan; China; India; Australia, South Korea, Mexico; Brazil; South Africa, UAE, Saudi Arabia
Key companies profiled
AngioDynamics; Medtronic; Teleflex Incorporated; Sciton, Inc.; Dornier Medtech; Merit Medical Systems; Alma Lasers; Biolitec AG; Boston Scientific Corporation
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Segments Covered in the Report
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2017 to 2030. For the purpose of this study, Grand View Research has segmented the global varicose veins treatment devices market report on the basis of type, and region:
-
Type Outlook (Revenue, USD Million, 2017 - 2030)
-
Endovenous Ablation
-
Endovenous Laser Therapy
-
Radiofrequency Ablation
-
-
Sclerotherapy
-
Surgical Ligation & Stripping
-
-
End Use Outlook (Revenue, USD Million; 2017 - 2030)
-
Vein Clinics
-
Hospitals
-
Ambulatory Care Unit
-
-
Regional Outlook (Revenue, USD Million, 2017 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
-
Latin America
-
Mexico
-
Brazil
-
-
Middle East & Africa (MEA)
-
South Africa
-
UAE
-
Saudi Arabia
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










