- Home
- »
- Market Trend Reports
- »
-
Emerging Trends In Kidney Disorder Management Devices
Executive Summary
The kidney disorder management devices industry is evolving with innovations in wearable dialysis, AI-driven systems, and remote monitoring. Emerging technologies enhance patient mobility, and home-based care. These advancements address the growing prevalence of CKD, improve outcomes, and open new opportunities for personalized, tech-integrated kidney care solutions
Technological Advancements
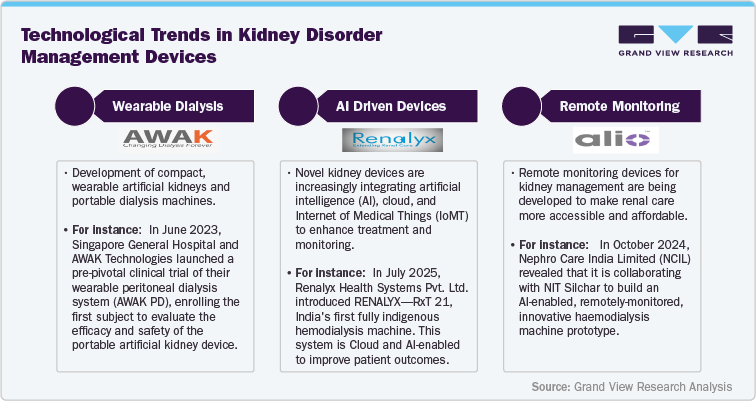
Market Drivers
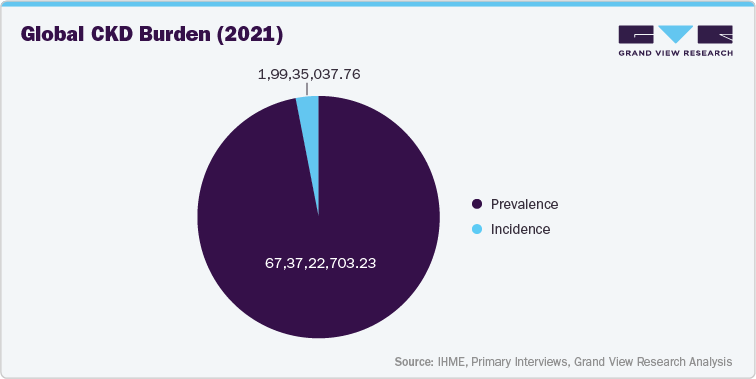
- RisingGlobal Prevalence of Kidney Disease and Associated Risk Factors
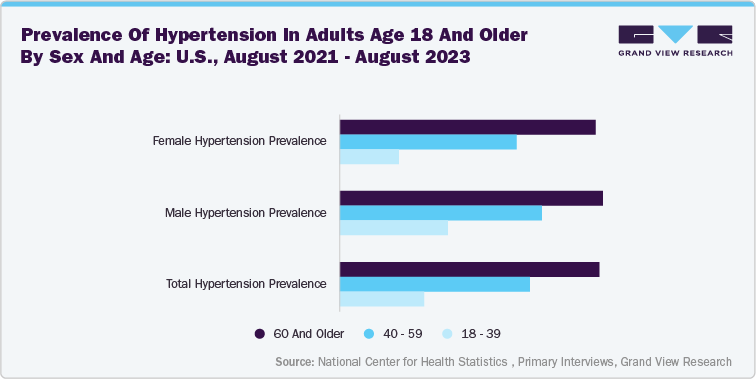
The increasing global burden of chronic kidney disease (CKD) is a primary driver behind the demand for advanced kidney disorder management devices. According to the data published by the National Kidney Foundation, 10% of the global population is impacted by chronic kidney disease. The prevalence is expected to rise further due to aging populations and the growing incidence of diabetes, hypertension, and cardiovascular diseases, all key risk factors for kidney dysfunction.
This surge in CKD cases is placing immense pressure on healthcare systems, creating a critical need for innovative devices that can support early diagnosis, ongoing monitoring, and more accessible treatment options such as portable or home-based dialysis. As a result, medical device companies are increasingly investing in technologies that can help manage CKD more effectively and improve patient outcomes.

-
Increasing Focus On Clinical Trials
There is a growing focus on developing novel devices for managing kidney disorders, which has increased research activity and clinical trials. Academic institutions, industry innovators, and regulatory bodies are forming partnerships to move wearable and portable artificial kidney technologies from prototype to clinical reality.
Below is a table summarizing a few of the recently completed or ongoing studies in this field.
Study Title
Conditions
Interventions
Sponsor
Enrollment
Completion Date
Primary Clinical Evaluation of Wearable Filtrating Artificial Kidney Device for On-site Medical Rescue
Fluid Overload
DEVICE: the wearable filtrating artificial Kidney Device|DEVICE: traditional hemodialysis machine
Chinese PLA General Hospital
12
12/31/2025
Late Feasibility Study to Evaluate Safety and Efficacy of AWAK PD Device in Subjects With ESKD.
Chronic Kidney Diseases
DEVICE: AWAK PD
AWAK Technologies Pte Ltd
12
7/29/2024
AI-Based Monitoring System for Chronic Heart Failure With Advanced Wearable and Mini-Invasive Devices
Chronic Heart Failure Cardiovascular Diseases|Heart Failure With Reduced Ejection Fraction (HFrEF)|Heart Failure With Preserved Ejection Fraction (HFPEF)|Congestive Heart Failure Chronic
DEVICE: Intervention Group (Device Group - AI-Based Remote Monitoring)|OTHER: Standard Clinical Follow-Up
University of Salerno
205
2/2/2027
AK+ Guard„ Pilot Study in Chronic Kidney Disease: Outpatient Diagnostic Accuracy and Remote Monitoring
Hyperkalemia|Chronic Kidney Disease (Stage 3-4)
DEVICE: AK+ Guard, ECG Application
AccurKardia, Inc.
50
11/28/2025
Source: ClinicalTrials.gov
Competitive Scenario
Some of the key players operating in the kidney disorder management devices include:
Baxter ., Fresenius Medical Care AG, Nipro Europe Group Companies , Vantive, ProKidney Corp, Vivance, Renalyx Health Systems Pvt Ltd , Asahi Kasei Medical Co., Ltd., Mozarc Medical (Medtronic), Pollet Medical Group, D.Med Healthcare GmbH & Co. KG, F. Hoffmann-La Roche Ltd, Dialyfix, B. Braun SE, NIKKISO Medical Europe GmbH

Recent Developments
Some of the strategies undertaken by key industry players operating in the kidney disorder management devices market are mentioned below:
-
In October 2025, Roche, in partnership with KlinRisk, Inc., secured CE‑mark approval for the first AI‑based risk stratification tool to predict progressive decline in kidney function. This new algorithm will be rolled out as part of Roche’s chronic kidney disease (CKD) algorithm panel, embedded in the navify Algorithm Suite, to assist clinicians in managing CKD across all stages of the disease.
-
In July 2025, Vantive, an organ‑therapy company, and Innovative Renal Care (IRC), which operates a broad network of kidney care programs across the U.S., signed a multi‑year strategic agreement. The collaboration aims to expand access to home dialysis therapies, enhance the patient therapy experience, and strengthen support for patients and care teams through improved technology‑enabled solutions.
-
In June 2025, Fresenius Medical Care, a provider of products and services for renal patients, entered the second phase of its U.S. rollout for high‑volume hemodiafiltration (HVHDF) therapy. The company recently secured FDA 510(k) approval for an enhanced version of its 5008X CAREsystem, now equipped with hemodiafiltration capability and additional features. This regulatory milestone lays the foundation for its expanded commercialization in 2025 and paves the way for a full commercial launch in 2026.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


