- Home
- »
- Market Trend Reports
- »
-
The MRI Market Opportunity - How Anti-Amyloid Therapies Are Driving New Imaging Needs
Overview of MRI In Neurodegenerative Disease Diagnosis
Magnetic resonance imaging (MRI) has become a cornerstone in the evaluation and management of neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), frontotemporal dementia (FTD), and other dementias. MRI provides a non-invasive, radiation-free method to visualize brain anatomy, quantify atrophy patterns, detect microvascular damage, and support differential diagnosis. With the rise of disease-modifying therapies (DMTs) for Alzheimer’s, especially anti-amyloid monoclonal antibodies, the role of MRI has expanded from diagnosis to continuous therapeutic monitoring, creating unprecedented demand for imaging infrastructure, advanced protocols, and AI-powered analysis tools.
Definition and Role of MRI in Alzheimer’s and Dementia Care
MRI is a non-invasive imaging modality that uses magnetic fields and radio waves to produce detailed brain images, allowing visualization of both grey and white matter. In Alzheimer’s and dementia care, MRI serves three principal roles:
-
Diagnostic differentiation - MRI helps distinguish Alzheimer’s disease from other dementias (vascular, frontotemporal, Lewy body) by identifying characteristic atrophy patterns.
-
Disease staging - Quantitative volumetric MRI measures hippocampal and cortical atrophy rates, correlating with cognitive decline.
-
Safety and monitoring - MRI is essential for monitoring treatment-related effects, particularly amyloid-related imaging abnormalities (ARIA) associated with anti-amyloid therapies such as lecanemab and donanemab.
MRI’s structural sensitivity supports both clinical diagnosis and longitudinal monitoring. T1-weighted imaging quantifies gray matter atrophy, FLAIR identifies white matter hyperintensities and edema, and susceptibility-weighted imaging (SWI) detects microhemorrhages crucial in identifying ARIA-H. As therapies transition from research to clinical use, serial MRIs are now embedded into treatment protocols, making MRI indispensable in modern dementia management.
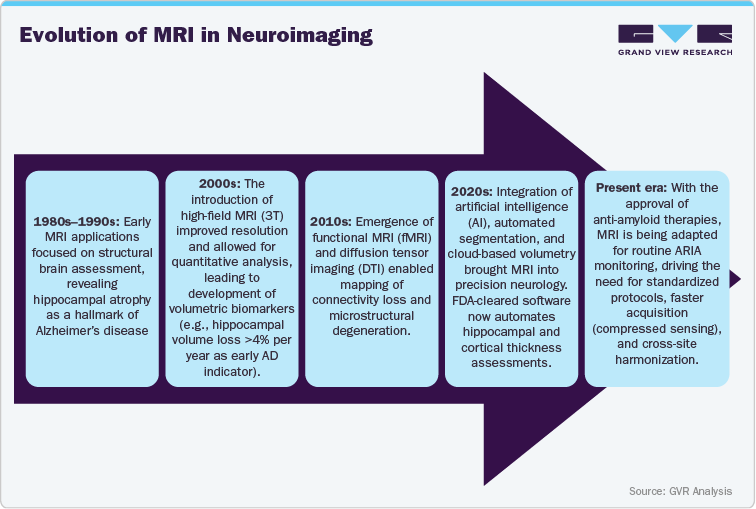
In parallel, quantitative MRI biomarkers (qMRI) and 7T ultra-high-field imaging are emerging to improve detection sensitivity and visualize microvascular amyloid burden. This progression underscores MRI’s transformation from a diagnostic tool to a central pillar of therapeutic safety and response evaluation.
MRI vs. PET in Amyloid Detection and Disease Monitoring
Although both MRI and positron emission tomography (PET) are central to Alzheimer’s diagnostics, they serve complementary purposes. PET visualizes molecular pathology (amyloid or tau), while MRI reveals structural and vascular effects. In the era of anti-amyloid therapeutics, MRI is preferred for safety surveillance and longitudinal follow-up, whereas PET is used for eligibility confirmation and treatment efficacy evaluation.
PET Visualizes Molecular Pathology
Aspect
MRI (Magnetic Resonance Imaging)
PET (Positron Emission Tomography)
Primary Function
Structural imaging; detects atrophy, edema, microbleeds
Molecular imaging; detects amyloid or tau deposits
Tracer/Contrast
None (non-invasive, radiation-free)
Requires radioactive tracers (e.g., 18F-florbetapir, 18F-flutemetamol)
Clinical Role in Alzheimer’s
Differential diagnosis; ARIA monitoring during therapy
Confirmation of amyloid pathology before therapy initiation
Utility in DMT Era
Essential for therapy safety monitoring (ARIA-E/H)
Used for therapy eligibility and efficacy endpoints
Frequency
Multiple serial scans (baseline + 3-6 follow-ups)
Baseline and occasional follow-up (due to radiation cost)
Cost and Accessibility
Lower per-scan cost; widely available
Higher cost; limited to major centers
Regulatory Role
Mandated in DMT safety protocols
Often required for treatment eligibility confirmation
Limitation
Cannot detect amyloid directly
Involves radiation exposure and tracer logistics
PET is ideal for detecting amyloid burden and confirming diagnosis, while MRI is indispensable for ongoing patient management especially to detect ARIA and evaluate neurodegeneration progression.
Role of MRI in the Era of Anti-Amyloid Therapeutics
With the arrival of anti-amyloid therapies such as Leqembi (lecanemab), Aduhelm (aducanumab), and donanemab, MRI has gained unprecedented clinical and commercial significance. Below are key roles MRI plays in this evolving treatment landscape:
1. Baseline Safety Assessment
-
MRI is mandatory before initiating therapy to exclude microhemorrhages or macrovascular pathology that could increase ARIA risk.
-
Identifies patients with pre-existing cerebral amyloid angiopathy or extensive white matter hyperintensities who require modified dosing or exclusion.
2. ARIA Surveillance
-
Anti-amyloid antibodies can cause ARIA-E (edema) or ARIA-H (microhemorrhages) in 20-35% of treated patients.
-
MRI, particularly FLAIR and SWI sequences, is the only modality capable of detecting these phenomena in real-time.
-
Current U.S. FDA guidelines recommend MRI at baseline, 3rd, 5th, 7th infusions, and if symptoms occur.
3. Longitudinal Disease Tracking
-
Serial MRI quantifies neurodegeneration over time, distinguishing true disease progression from treatment side effects.
-
AI-based tools provide volumetric trend reports for clinicians and researchers, improving sensitivity to small structural changes.
4. Clinical Trial and Real-World Integration
-
In clinical trials, MRI endpoints have become standard for both efficacy (atrophy rates) and safety (ARIA monitoring).
-
In post-approval registries, MRI data are integrated into pharmacovigilance systems and real-world evidence platforms.
5. Technological Innovation Catalyst
-
The surge in MRI demand is prompting hospitals to upgrade scanners (from 1.5T to 3T), add dedicated neuroimaging coils, and adopt faster imaging protocols.
-
Vendors like Siemens Healthineers, GE HealthCare, Philips, Canon Medical, and United Imaging are launching neuro-optimized MRI systems and AI software designed for dementia care.
Key Market Drivers and Trends
Regulatory Approval: Leqembi (lecanemab-irmb)
Indication & mechanism
Leqembi (lecanemab-irmb), developed by Eisai Co., Ltd. and Biogen Inc., is a humanised IgG1 monoclonal antibody directed against aggregated soluble Aβ protofibrils and insoluble Aβ fibrils, key components of amyloid-β pathology in Alzheimer’s disease.
U.S. FDA milestones
-
January 6, 2023 - Leqembi received accelerated approval from the U.S. Food and Drug Administration (FDA) for the treatment of early Alzheimer’s disease (mild cognitive impairment or mild dementia stage) in patients with confirmed amyloid-β pathology.
-
July 6, 2023 - The FDA granted traditional (full) approval to Leqembi, making it the first anti-amyloid antibody therapy to be converted from accelerated to full approval after confirmatory trial results.
-
January 26, 2025 - The FDA approved a maintenance dosing regimen (10 mg/kg once every four weeks) for Leqembi in adults with early Alzheimer’s disease.
-
August 29, 2025 - The FDA approved a subcutaneous autoinjector version of Leqembi (Leqembi IQLIK) for maintenance dosing in early Alzheimer’s disease.
The approved population: adults with early Alzheimer’s disease (MCI or mild dementia) and confirmed amyloid pathology, matching the enrolment criteria of pivotal trials (e.g., CLARITY AD Study 301, n=1,795) which demonstrated a statistically significant reduction in clinical decline.
Safety warnings include risk of amyloid-related imaging abnormalities (ARIA-E: edema/effusion and ARIA-H: microhemorrhages) MRI surveillance is mandated as part of monitoring protocols.
Regulatory Approval: Kisunla (donanemab-azbt)
Indication & mechanism
Kisunla (donanemab-azbt), developed by Eli Lilly and Company, is a humanised IgG1 monoclonal antibody targeting a pyroglutamate form of amyloid-β (Aβp3-42) that aggregates into plaques. The indication focuses on adults with early symptomatic Alzheimer’s because of amyloid pathology.
U.S. FDA milestones
-
July 2, 2024 - The FDA approved Kisunla for the treatment of early symptomatic Alzheimer’s disease (mild cognitive impairment or mild dementia stage), in patients with confirmed amyloid pathology. The pivotal TRAILBLAZER-ALZ2 Phase 3 trial (n=1,736) showed up to ~35% slowing of cognitive/functional decline in eligible patients.
European Medicines Agency (EMA) milestones
-
March 27, 2025 - Initially, the EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended against marketing authorization due to ARIA risk vs. benefit concerns.
-
July 25, 2025 - Following re-examination, the CHMP issued a positive opinion recommending approval of Kisunla for adult patients with early Alzheimer’s disease who are ApoE ε4 heterozygotes or non-carriers and have confirmed amyloid pathology. The final decision rests with the European Commission.
The restriction to patients with one or no copy of the ApoE ε4 gene (due to elevated ARIA risk in ε4 homozygotes) significantly narrows the eligible patient population in the EU setting.
The indication and label emphasize infusion administration every four weeks and underscore the need for brain MRI monitoring for safety.
Market Challenges and Restraints
The MRI market linked to anti-amyloid therapies faces several challenges that impact on its adoption and scalability:
1. Regulatory Uncertainties and Country-Specific Approvals:
Diverse regulatory standards across the U.S., EU, and Asia delay global harmonization. MRI protocols for ARIA detection and reimbursement eligibility vary by region, creating compliance and approval hurdles.
2. Variability in Injector Skill and Training:
While not injectors, the parallel challenge lies in technologist and radiologist expertise. Differences in MRI interpretation skills for ARIA detection can affect diagnostic accuracy and treatment monitoring consistency.
3. Limited Clinical Evidence and Long-Term Data:
There’s insufficient long-term data validating MRI’s predictive role in monitoring anti-amyloid therapy outcomes. Ongoing trials focus on PET or biomarker-based tracking, leaving MRI’s full clinical value under-documented.
4. Product Commoditization and Pricing Pressure:
As MRI hardware becomes standardized, vendors face pricing pressure. Hospitals seek cost-effective AI or software-based upgrades rather than full system replacements, limiting premium system sales.
5. Safety Concerns and Adverse Event Reporting:
Anti-amyloid therapies increase the risk of ARIA, requiring frequent MRI scans. However, repeated imaging, contrast use, and workload strain radiology departments, emphasizing the need for safety protocols and improved adverse event tracking.
Technological Innovations in MRI for Neurodegenerative Disease Imaging
1. Advanced Neuroimaging Sequences (FLAIR, SWI, DTI):
Modern MRI sequences such as FLAIR (Fluid-Attenuated Inversion Recovery), SWI (Susceptibility-Weighted Imaging), and DTI (Diffusion Tensor Imaging) are revolutionizing neurodegenerative diagnostics. These techniques improve detection of Amyloid-Related Imaging Abnormalities (ARIA), microbleeds, and white matter integrity changes associated with Alzheimer’s and other dementias.
2. Volumetric and Structural Brain Analysis Tools:
AI-assisted volumetric tools like NeuroQuant, FreeSurfer, and Brainreader’s Neuroreader enable automated brain volume measurement and atrophy tracking. These systems quantify hippocampal shrinkage and cortical thinning critical indicators of disease progression in patients on anti-amyloid therapy.
3. MRI-Compatible Contrast Agents for Amyloid Plaque Visualization:
Emerging gadolinium-free contrast agents and targeted molecular probes are enhancing amyloid plaque visualization without the risks of nephrogenic systemic fibrosis. Research focuses on developing agents that can bind to amyloid aggregates, providing PET-like specificity with MRI safety and accessibility.
4. Quantitative MRI and Machine Learning in Biomarker Detection:
Quantitative MRI (qMRI) integrates AI and machine learning algorithms to identify microstructural changes, such as myelin loss or iron deposition, serving as non-invasive biomarkers. AI-driven pattern recognition improves diagnostic accuracy and supports personalized monitoring of treatment response.
5. Cloud-Based MRI Data Management Platforms:
Cloud-enabled MRI platforms allow remote data sharing, AI analysis, and cross-site collaboration. Vendors like Siemens Healthineers (teamplay), GE Healthcare (Edison AI), and Philips (HealthSuite) are deploying secure cloud ecosystems that enhance scalability and accelerate multicenter trials for anti-amyloid drug efficacy.
Clinical Applications and Use Cases
MRI in Patient Stratification for Anti-Amyloid Therapies
MRI plays a vital role in identifying eligible patients for anti-amyloid treatments like Leqembi (lecanemab) or Kisunla (donanemab). For example, before starting therapy, clinicians use MRI to rule out extensive microhemorrhages or brain edema, which may increase the risk of treatment-related complications. Patients showing early Alzheimer’s biomarkers and mild brain atrophy but without severe vascular damage are typically cleared for therapy.
Example: At Mass General Hospital (U.S.), MRI-based screening is used to select Alzheimer’s patients for lecanemab therapy, helping determine who will respond best and minimizing ARIA risk.
Monitoring ARIA (Amyloid-Related Imaging Abnormalities)
After initiating anti-amyloid therapy, MRI is the gold standard for detecting ARIA-E (edema) and ARIA-H (microhemorrhages) side effects linked to amyloid clearance. Regular MRI scans (typically every 3-6 months) help clinicians track and manage ARIA progression without interrupting effective therapy.
Example: In the CLARITY-AD trial of lecanemab, MRI was used at baseline and periodically to monitor ARIA events, allowing early detection of asymptomatic abnormalities that could otherwise escalate into neurological complications.
MRI-Guided Precision Medicine in Neurology
MRI enables personalized treatment planning by visualizing structural and functional brain changes. Combining MRI data with cognitive assessments and genetic markers (like APOE ε4) allows neurologists to customize dosing or combine anti-amyloid therapy with lifestyle or cognitive interventions.
Example: At the Mayo Clinic Alzheimer’s Center, MRI findings are integrated with genetic and fluid biomarkers to tailor individual treatment pathways, optimizing outcomes and reducing unnecessary drug exposure.
Competitive Landscape and Company Profiles
Company
Recent Developments
Description
Eli Lilly and Company
September 2025
Received European Commission marketing authorization for its Alzheimer’s therapy Kisunla (donanemab) for early symptomatic Alzheimer’s disease this heightens MRI demand for monitoring ARIA and treatment follow-up.
GE HealthCare
September 2025
Announced acquisition of AI brain-imaging company icometrix. This integrates icometrix’s icobrain ARIA-detection software (FDA-cleared) with GE MRI systems, targeting enhanced imaging workflows for Alzheimer’s and anti-amyloid therapeutic monitoring.
Siemens Healthineers AG
October 2024
Received FDA clearance for two new Alzheimer’s disease-analysis features in its syngo.PET Cortical Analysis software (Centiloid amyloid quantification and tau PET quantification). While PET-centric, the company also markets MRI protocols for ARIA monitoring, linking MRI growth to Alzheimer’s therapy rollout.
Life Molecular Imaging (LMI)
May 2025
Secured a USD 2.16 million investment from the Alzheimer’s Drug Discovery Foundation (ADDF) to advance development of an investigational PET tracer ([18F]F-DED) for neuroinflammation in Alzheimer’s disease, complementing structural imaging such as MRI.
Lantheus Holdings, Inc.
Jan 13 2025
Announced acquisition of Life Molecular Imaging for an upfront USD 350 million (plus earn-outs) to build its Alzheimer’s disease radiodiagnostics franchise (e.g., Neuraceq amyloid PET). This augments the broader imaging ecosystem (PET + MRI) supporting anti-amyloid therapies.
Share this report with your colleague or friend.
Pricing & Purchase Options
Service Guarantee
-
Insured Buying
This report has a service guarantee. We stand by our report quality.
-
Confidentiality
We are in compliance with GDPR & CCPA norms. All interactions are confidential.
-
Custom research service
Design an exclusive study to serve your research needs.
-
24/5 Research support
Get your queries resolved from an industry expert.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


