- Home
- »
- Market Trend Reports
- »
-
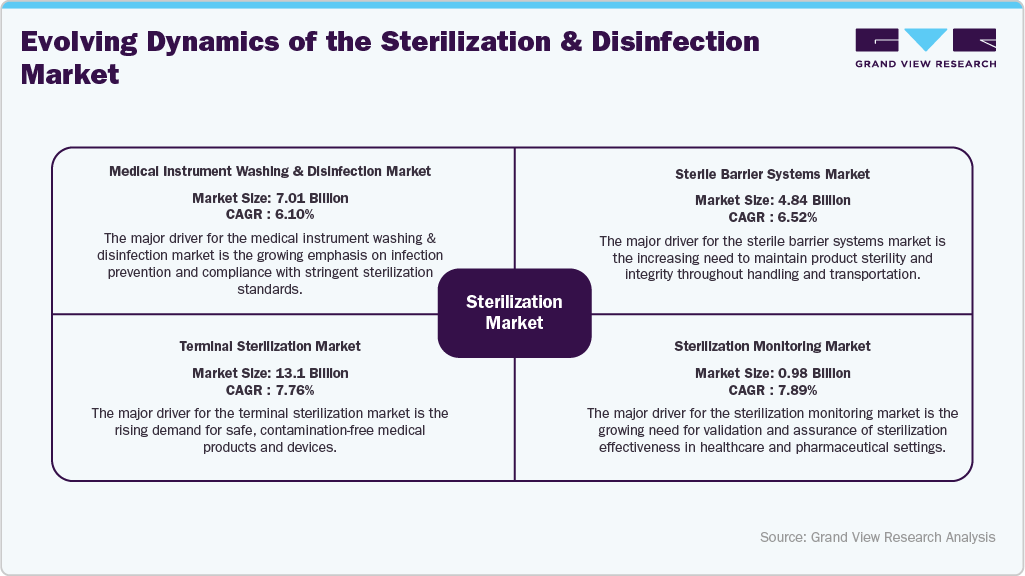
Evolving Dynamics of the Sterilization & Disinfection Market
Executive Summary
The global sterilization process trend report unites insights from sterilization monitoring, terminal sterilization, medical instrument washing & disinfection, and sterile barrier systems markets. It highlights a growing shift toward automation, digital monitoring, and sustainable sterilization practices driven by stricter infection control standards and rising healthcare demand. Innovations such as IoT-enabled validation systems, eco-friendly sterilant, and advanced sterile barriers are transforming sterility assurance into a more connected, efficient, and compliant ecosystem, marking the emergence of next-generation Sterility.
Key Segments And Growth Drivers Of The Sterilization Market
Opportunities for Sterilization and Disinfection Market:
-
Approvals of HLD Products And Consumables:
The increasing number of regulatory approvals for high-level disinfection (HLD) consumables and systems creates strong opportunities for product portfolio expansion among industry participants. Global health authorities such as the U.S. FDA, European Medicines Agency (EMA), and regional regulators support the introduction of new HLD formulations with enhanced efficacy against a broader range of pathogens, including bacterial spores. This regulatory momentum prompts hospitals and outpatient surgical centers to modernize their disinfection protocols to meet evolving accreditation and infection control standards. For instance, in February 2025, the Association for the Advancement of Medical Instrumentation (AAMI) and the American National Standards Institute (ANSI) updated standard ST58:2024 to include chlorine dioxide foam as an approved high-level disinfectant for medical devices. The update also highlights FDA-cleared innovations such as Parker Laboratories’ Tristel ULT system, which enhances ultrasound probe disinfection and residue removal for improved safety and efficiency. Consequently, manufacturers are given opportunities to reformulate and validate washer-disinfectant systems compatible with these next-generation HLD agents, broadening their range of compliant, effective, and high-performance solutions in the market.
-
Technological Innovations:
Technological advancements in sterilization monitoring are unlocking major market opportunities by improving speed, automation, and digital connectivity. Industry players are introducing next-generation systems designed to enhance efficiency and compliance. For instance, in August 2025, ASP launched the BIOTRACE Instant Read Steam System, the fastest FDA-cleared biological indicator in the U.S., providing sterilization verification in just seven seconds compared to conventional 20-minute systems. Tailored for sterile processing departments (SPDs), the compact device features dual test positions, data storage for the last 50 results, and multi-mode connectivity via USB, Wi-Fi, and Bluetooth. Integrated with the SM Cloud Web Application and BIOTRACE Assist App, it enables secure cloud data storage, automated documentation, and synchronization with hospital instrument tracking systems such as CensiTrac. Leveraging advanced fluorescence detection, the system ensures rapid and precise readouts. These innovations empower healthcare facilities to minimize turnaround times, boost throughput, and streamline sterilization workflows, creating strong growth opportunities for manufacturers to expand and differentiate their sterilization monitoring portfolios.
“The launch of BIOTRACE Instant Read Steam System represents a significant advancement in our ability to deliver fast, actionable sterility data to frontline staff; By reducing the readout time to just seconds, we’re addressing a long-standing bottleneck that often impacts both productivity and peace of mind. This system supports SPDs not only in meeting compliance standards, but in exceeding them, with confidence. Said Dr. Ivan Salgo, Vice President and Chief Medical and Scientific Officer at ASP.
- Rising Demand For Automated Disinfection:
The rising demand for automated disinfection systems is opening significant growth opportunities for manufacturers in the medical instrument washing & disinfection market. Increasing procedural volumes, staffing shortages, and growing compliance pressures are pushing healthcare facilities to adopt automated workflows that reduce human error and variability in reprocessing. Automated washers and disinfectors with programmable cycles and integrated chemical dosing features are becoming essential for Central Sterile Supply Departments (CSSDs) and outpatient surgical centers. For instance, in July 2025, Nanosonics launched trophon3, an automated ultrasound probe disinfection device that was over 40% faster than previous models. Initially released in Europe, the UK, Australia, and New Zealand, it featured enhanced digital integration and traceability, including compatibility with DICOM imaging data systems.
"The launch of trophon3 and trophon2 Plus set a new benchmark in automated HLD for ultrasound transducers, upholding the trophon technology's market leadership position. These launches reflect our commitment to innovation, customer support and ongoing expansion in this important area of infection prevention," said Michael Kavanagh, CEO and President.
Cross-Segment Analysis: Sterilization and Disinfection Market
-
Interconnected Ecosystem:
The sterilization process market functions as a unified value chain, where four key segments-Medical Instrument Washing & Disinfection, Sterile Barrier Systems, Terminal Sterilization, and Sterilization Monitoring-work together to ensure complete infection prevention across healthcare and medical device settings.
-
Medical Instrument Washing & Disinfection:
Acts as the foundation of the sterilization workflow. Automated washer-disinfectors, enzymatic detergents, and high-level disinfectants remove organic residues and microbial contaminants before sterilization. Widely used in CSSDs, outpatient surgical centers, and diagnostic labs for pre-sterilization cleaning and decontamination.
-
Sterile Barrier Systems:
Following cleaning, instruments are enclosed in sterile pouches, wraps, or rigid containers that preserve sterility during handling, transport, and storage. These systems are essential for medical device manufacturers and hospital sterilization units, ensuring microbial protection and packaging integrity until use.
-
Terminal Sterilization:
Employs advanced methods such as steam, ethylene oxide (EtO), gamma irradiation, and hydrogen peroxide to achieve complete microbial inactivation. This process is critical for both reusable hospital instruments and single-use medical devices, ensuring patient safety and product sterility before market release.
-
Sterilization Monitoring:
Incorporates biological indicators, chemical indicators, and digital verification tools to validate sterilization efficacy and maintain process traceability. Used by hospitals, contract sterilization providers, and device manufacturers to meet stringent regulatory compliance and documentation standards.
Competitive Landscape: Sterilization and Disinfection Market - Key Players, Innovations & Recent Launches
Company
Products
Recent Developments
New Launches
STERIS
V-PRO Low Temperature Sterilizer
STERIS plc has completed the acquisition of BD’s surgical and laparoscopic instrumentation, along with its sterilization container assets
V-PRO 60 Low Temp System
3M
3M Attest Rapid Readout Biological Indicator
Launch of Attest Super Rapid Vaporized Hydrogen Peroxide (VH2O2) Clear Challenge Pack
Launch of Attest Super Rapid Vaporized Hydrogen Peroxide (VH2O2) Clear Challenge Pack
Advanced Sterilization Sterilization Products (ASP)
STERRAD 100NX System with ALLClear Technology
Advanced Sterilization Products (ASP) expanded its Sterilization Monitoring portfolio with new steam monitoring products to enhance sterility assurance and operational efficiency.
Launch ASP Sterrad 200NX Low Temp System
Shinva Medical Instrument Co., Ltd.
Automatic Flexible Endoscope Washer-disinfector
Entered Southeast Asian market
Shinva 1000L Large Volume Autoclave
Getinge
GTS Steam Air Mix Sterilizer
Getinge has completed the acquisition of Ultra Clean Systems Inc., a U.S.-based leader in ultrasonic cleaning technologies used for surgical instrument decontamination in hospitals and surgery centers.
Getinge launched the Poladus 150, a next-generation low-temperature sterilizer for heat-sensitive surgical instruments, featuring advanced cross-contamination barrier technology to reduce healthcare-associated infection risks.
Source: Grand View Research Analysis
Some of the key players operating in the sterilization and disinfection market include:
-
ASP (Fortive)
-
STERIS
-
MELAG Medizintechnik GmbH & Co. KG
-
Tuttnauer
-
Olympus
-
ECOLAB
-
Shinva Medical Instrument Co., Ltd.
-
Getinge
-
Skytron, LLC
-
AT-OS S.r.l.
-
COLTENE Group
-
Map Industries
-
Spire Integrated Solutions
-
Steelco S.p.A.
-
Belimed
-
Smeg
-
TBT Medical
-
DEKO MedTech Oy.
-
MMM Group
-
Nanosonics
-
SCHLUMBOHM Medizin-Labor-Technologie-Hamburg GmbH
-
KEN Hygiene Systems
-
Germitec
-
CS Medical LLC
-
Metrex Research, LLC
-
CIVCO Medical Solutions
-
Tristel Plc
Recent Developments
Some of the strategies undertaken by key industry players operating in the sterilization and disinfection market are mentioned below:
-
In August 2025, Nanosonics has received approval from the U.S. Food and Drug Administration (FDA) for its latest ultrasound probe disinfection technologies-trophon3 and the trophon2 Plus software upgrade-paving the way for their commercial launch across the US healthcare sector. This regulatory approval enables Nanosonics to expand into US hospitals and private clinics, which collectively represent approximately 30,000 potential new device installations. The new trophon3 system offers several improvements over previous models, including a disinfection cycle that is over 40% faster, enhanced digital integration capabilities, and expanded traceability features.
-
In June 2025, Solventum, one of the global leaders in MedTech innovation focused on infection prevention, announced the launch of its Attest Super Rapid Vaporized Hydrogen Peroxide (VH2O2) Clear Challenge Pack. This ready-to-use test combines two FDA-cleared indicators- a biological indicator (BI) that confirms microbial neutralization and a chemical indicator (CI) that verifies proper sterilizer performance- into a single-use pack featuring a transparent container.
-
In March 2024, Getinge's launch of a new, intuitive, high-performance multi-chamber washer-disinfector is a significant development in the infection control sector, aimed at addressing the need for efficient, high-capacity cleaning in healthcare facilities. Multi-chamber washer disinfectors are particularly valuable in hospitals and large medical centers where large volumes of surgical instruments and medical equipment must be sterilized quickly and effectively.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


