- Home
- »
- Market Trend Reports
- »
-
Tolebrutinib (BTK Inhibitor) In Multiple Sclerosis: Clinical Development, Regulatory Status, And Market Overview
Overview
Tolebrutinib, developed by Sanofi, is an innovative oral Bruton's tyrosine kinase (BTK) inhibitor targeting multiple sclerosis (MS), a chronic autoimmune disease affecting approximately 2.5 million people worldwide. By selectively modulating neuroinflammatory pathways, Tolebrutinib addresses both relapsing and progressive forms of MS, including non-relapsing secondary progressive MS (nrSPMS), a population with limited treatment options. The drug has demonstrated significant efficacy in delaying disability progression, with meaningful reductions in confirmed disability progression observed in clinical trials. Currently under FDA and EMA review, Tolebrutinib is poised to become the first marketed BTK inhibitor for MS, offering a promising alternative to existing therapies and potentially redefining progressive MS management.
Key Report Deliverables
-
Analyze the Tolebrutinib market landscape, detailing its current status, ongoing clinical development, anticipated market entry, and key trends shaping the multiple sclerosis (MS) treatment segment.
-
Evaluate the competitive environment, identifying key players such as Sanofi, Biogen (Tecfidera), Novartis (Mayzent), and emerging pipeline candidates, along with strategic partnerships and market positioning.
-
Forecast market growth, projecting adoption trends, patient population expansion, and geographic penetration across relapsing and progressive MS segments, while assessing potential risks to growth.
-
Identify regulatory and market barriers, providing insights into approval timelines, pricing, reimbursement challenges, and safety monitoring requirements that could impact market expansion.
-
Concurrent Competitive Landscape, examining both direct and indirect competitors, differentiating therapeutic profiles, and analyzing strategic moves shaping the evolving MS treatment market.
Transforming The Future Of Multiple Sclerosis Treatment
Tolebrutinib, developed by Sanofi, is emerging as a breakthrough therapy for the management of multiple sclerosis (MS), a chronic autoimmune disorder characterized by immune-mediated damage to the central nervous system. MS affects approximately 2.5 million individuals globally and can lead to symptoms such as muscle weakness, fatigue, impaired coordination, and cognitive dysfunction, significantly impacting quality of life. Traditional treatment options, including injectable disease-modifying therapies and monoclonal antibodies, often reduce relapse rates but fail to address the underlying neuroinflammatory mechanisms driving disease progression. Tolebrutinib, as a selective Bruton's tyrosine kinase (BTK) inhibitor, offers a novel therapeutic approach by modulating key immune pathways, targeting the root cause of MS rather than merely controlling symptoms.
Tolebrutinib’s mechanism of action focuses on inhibiting BTK signaling in B cells and myeloid cells, thereby reducing neuroinflammation, demyelination, and subsequent axonal damage. This targeted approach slows disability progression, decreases relapse frequency, and may preserve neurological function, which is particularly critical in patients with relapsing-remitting MS (RRMS) and non-relapsing secondary progressive MS (nrSPMS). Unlike traditional therapies, Tolebrutinib is an oral therapy that allows for convenient dosing and consistent pharmacologic activity, potentially improving patient adherence and long-term outcomes. By directly addressing the molecular drivers of MS progression, Tolebrutinib is poised to transform disease management, offering improved neurological function, symptom control, and potential long-term disease-modifying benefits.
Currently in late-stage clinical development, Tolebrutinib has demonstrated promising efficacy and safety across multiple patient populations, signaling its potential as a first-in-class BTK inhibitor for MS. The drug is under regulatory review in key markets, including the U.S., Europe, and Asia-Pacific regions, and is expected to provide a novel oral treatment option for patients with limited alternatives. Its development marks a significant milestone in precision neuroimmunology, reflecting a shift toward targeted, mechanism-based therapies that go beyond symptomatic management. With increasing disease awareness, unmet clinical needs, and growing demand for oral therapies, Tolebrutinib is well-positioned to reshape the global MS treatment landscape and improve outcomes for patients living with this complex neurological disorder.

Current Market Scenarios
The global multiple sclerosis (MS) therapeutics landscape is evolving rapidly, driven by increasing disease awareness, earlier diagnosis, and rising demand for innovative treatment options. Traditional therapies, including injectable disease-modifying treatments and monoclonal antibodies, primarily reduce relapse frequency but do not fully address the underlying neuroinflammatory mechanisms driving disease progression. In this context, Tolebrutinib, an oral Bruton's tyrosine kinase (BTK) inhibitor developed by Sanofi, represents a novel therapeutic approach. By selectively modulating B cell and myeloid cell activity, it targets the root causes of MS, reducing neuroinflammation and slowing disability progression. Its development reflects a shift toward precision neuroimmunology and mechanism-based treatment strategies in neurological medicine.
Recent clinical trials, including the HERCULES and TOPAZ studies, have highlighted Tolebrutinib’s efficacy and safety profile in patients with relapsing-remitting MS (RRMS) and non-relapsing secondary progressive MS (nrSPMS). Results demonstrated significant reductions in relapse rates, delayed disability progression, and improvements in MRI-based disease activity compared to standard therapies. The oral, once-daily dosing regimen enhances patient convenience and adherence, distinguishing it from injectable therapies that may pose administration challenges and systemic side effects. Both RRMS and nrSPMS patients stand to benefit from this targeted approach, which has been recognized by neurologists for its potential to transform disease management.
Looking ahead, Tolebrutinib is expected to reshape the global MS market, driven by unmet clinical needs, favorable trial outcomes, and strategic collaborations for international commercialization. Partnerships and licensing agreements have expanded their reach, reinforcing their commercial potential and anticipated adoption across major markets. Its mechanism-based approach represents a milestone in precision neuroimmunology, promising long-term benefits in disease management and patient quality of life. As the market shifts toward targeted oral therapies, Tolebrutinib is positioned to set a new standard of care, offering clinicians a more effective and convenient alternative to traditional treatments, and potentially redefining therapeutic expectations for multiple sclerosis patients worldwide.
Targeted BTK Inhibition for Neuroimmunological Precision
Tolebrutinib is a selective Bruton's tyrosine kinase (BTK) inhibitor designed to directly modulate the molecular mechanisms driving multiple sclerosis (MS). In MS, immune-mediated activation of B cells and myeloid cells contributes to neuroinflammation, demyelination, and subsequent axonal damage, leading to neurological disability and functional decline. Tolebrutinib binds specifically to BTK in these immune cells, inhibiting downstream signaling pathways that trigger inflammatory cascades and central nervous system (CNS) injury. By targeting this fundamental pathological process, the drug addresses the root cause of MS rather than merely mitigating symptoms. This mechanism allows Tolebrutinib to provide precise control over neuroinflammatory activity, reducing disease progression while preserving neurological function, differentiating it from conventional therapies like injectable disease-modifying agents or monoclonal antibodies.
The reduction of neuroinflammation is a key benefit of Tolebrutinib. By suppressing overactive B cell and myeloid cell signaling, it slows the formation of new lesions, preserves myelin integrity, and protects neuronal pathways. This results in delayed disability progression, fewer relapses, improved cognitive and physical function, and a lower risk of long-term neurological deterioration. Unlike traditional therapies, which often have systemic immunosuppressive effects or require invasive administration, Tolebrutinib’s targeted oral approach ensures a tunable, patient-specific therapeutic effect, optimizing both safety and efficacy. Its ability to modulate key immune pathways while minimizing off-target effects highlights its potential as a disease-modifying therapy rather than solely a symptomatic treatment.
Tolebrutinib’s mechanism offers advantages over conventional MS treatments by combining efficacy with precision. Standard therapies, while effective in reducing relapse frequency, do not fully prevent neurodegeneration or halt disease progression. In contrast, Tolebrutinib directly intervenes at the molecular level, potentially preserving long-term neurological function. Its selective action minimizes adverse effects often seen with broad immunosuppressive agents, while the oral, once-daily dosing regimen allows clinicians to optimize therapy based on individual patient response, providing a personalized treatment approach. By addressing both relapsing-remitting MS (RRMS) and non-relapsing secondary progressive MS (nrSPMS), Tolebrutinib represents a new era in precision neuroimmunology, offering improved patient outcomes and enhanced quality of life.
Clinical Development and Trial Insights
Tolebrutinib’s clinical development has been highlighted by robust studies, including the Phase II HERCULES and Phase III TOPAZ trials, providing critical insights into its safety, efficacy, and therapeutic potential in multiple sclerosis (MS). The Phase II HERCULES trial focused on patients with relapsing-remitting MS (RRMS), evaluating the drug’s ability to reduce neuroinflammation, lesion formation, and relapse rates. Results showed consistent reductions in MRI-based disease activity, delayed disability progression, and improvements in neurological function. Adverse events were mostly mild to moderate, reflecting a favorable safety profile. These findings emphasize Tolebrutinib’s potential as an effective alternative to traditional therapies, which generally reduce relapse frequency but do not fully address the underlying pathophysiology of MS.
The Phase III TOPAZ trial further assessed Tolebrutinib in a broader patient population, including non-relapsing secondary progressive MS (nrSPMS). The study confirmed reductions in lesion accumulation, preservation of myelin integrity, and slower disability progression, demonstrating the drug’s mechanism-based effect. Dose optimization was a central aspect, showing that individualized titration maximizes therapeutic benefit while minimizing risks such as systemic immunosuppression or infection. This precise, patient-specific dosing reinforces Tolebrutinib’s role as a personalized therapy, allowing clinicians to tailor treatment based on individual patient response, which is crucial for managing a heterogeneous condition like multiple sclerosis.
Data from the Phase II and Phase III trials strongly support Tolebrutinib as a potential first-in-class BTK inhibitor for MS. Evidence demonstrates improvements in relapse reduction, neurological function, and quality of life, addressing the limitations of existing treatments. By directly targeting B cell and myeloid cell signaling, Tolebrutinib also shows promise in slowing long-term disease progression, a benefit not provided by conventional therapies. The consistent safety profile, flexible oral dosing regimen, and efficacy across RRMS and nrSPMS populations establish a strong foundation for regulatory approvals and commercial adoption, positioning Tolebrutinib as a transformative agent in the emerging field of precision neuroimmunology.

Regulatory Milestones and Commercial Readiness
Tolebrutinib has made significant progress in its regulatory journey, reflecting its potential as a first-in-class BTK inhibitor for multiple sclerosis (MS). The Phase III TOPAZ and Phase II HERCULES trial data supported submission of the New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA). The FDA has set a target action date for review, and approval is anticipated in the near future, positioning Tolebrutinib for commercial launch in the United States shortly thereafter. Regulatory filings in Europe and Asia-Pacific regions are also underway, following positive trial outcomes and established safety and efficacy profiles. The structured regulatory strategy underscores Sanofi’s commitment to achieving timely global access for patients with both relapsing-remitting MS (RRMS) and non-relapsing secondary progressive MS (nrSPMS).
Commercial readiness for Tolebrutinib is supported by robust strategic planning and market preparation. Sanofi has entered partnerships to enhance regional distribution and maximize reach, including collaborations for Japan and select Asia-Pacific markets. These agreements facilitate regulatory submissions, local manufacturing, and market access strategies, ensuring seamless entry upon approval. Marketing and education initiatives are being designed to train neurologists and healthcare providers on patient selection, dose optimization, and safety monitoring. By aligning regulatory approvals with commercial strategies, Sanofi aims to rapidly position Tolebrutinib as a leading therapy in the MS treatment landscape, emphasizing precision neuroimmunology, oral administration, and patient-centered outcomes.
Global commercialization of Tolebrutinib is expected to benefit from growing awareness of MS and the unmet need for effective, mechanism-based therapies. Launch strategies are being tailored to each region, considering local healthcare infrastructure, reimbursement policies, and market dynamics. Europe and Asia-Pacific markets are prioritized for early adoption, leveraging strategic partnerships to ensure regulatory compliance and smooth product rollout. Commercial readiness includes pre-launch medical education programs, patient support initiatives, and distribution logistics. By integrating regulatory milestones with global market strategies, Sanofi aims to optimize adoption, achieve strong market penetration, and establish Tolebrutinib as a standard-of-care therapy. This coordinated approach reflects a comprehensive plan for long-term success in precision neuroimmunology.
Market Dynamics and Growth Drivers
The multiple sclerosis (MS) market is expanding due to the increasing prevalence of the disease and rising global awareness. MS is a chronic autoimmune disorder affecting millions worldwide, many of whom remain undiagnosed or misdiagnosed due to subtle or variable neurological symptoms. Advances in diagnostic techniques, including MRI imaging, biomarker testing, and early neurologic assessments, are improving timely detection and enabling intervention at earlier stages. Early diagnosis allows patients to benefit from novel therapies like Tolebrutinib, which directly target the underlying neuroinflammatory mechanisms. The growing population of diagnosed patients, combined with improved access to healthcare, is creating a strong demand for innovative treatments that go beyond symptomatic management.
Unmet clinical needs are a major driver of market growth, as traditional MS therapies primarily reduce relapse frequency without fully preventing disease progression or long-term neurological damage. Injectable disease-modifying therapies and monoclonal antibodies provide limited efficacy in progressive forms of MS and often carry risks of adverse effects. Tolebrutinib, as a precision-targeted Bruton's tyrosine kinase (BTK) inhibitor, addresses B cell and myeloid cell overactivity, reduces neuroinflammation, and slows disability progression, offering a mechanism-based approach. Clinicians are increasingly seeking therapies that can improve patient outcomes, preserve neurological function, and enhance quality of life. The recognition of these unmet needs has accelerated research and development efforts, positioning Tolebrutinib to fill a critical gap in MS treatment.
The growing focus on precision neuroimmunology further supports market expansion, as healthcare systems emphasize personalized and targeted treatments. Tolebrutinib’s oral, once-daily dosing and ability to be optimized based on individual patient response exemplify this trend, allowing for improved adherence and minimized risks. In addition, patient education programs, early detection initiatives, and updated clinical guidelines are facilitating wider adoption of advanced therapies. Strategic collaborations and partnerships in key regions are also enhancing market access, ensuring the drug reaches patients efficiently. Collectively, these factors-rising prevalence, unmet clinical needs, improved diagnostics, and the shift toward mechanism-based precision therapies-are driving robust growth and shaping the future landscape of the MS therapeutics market.
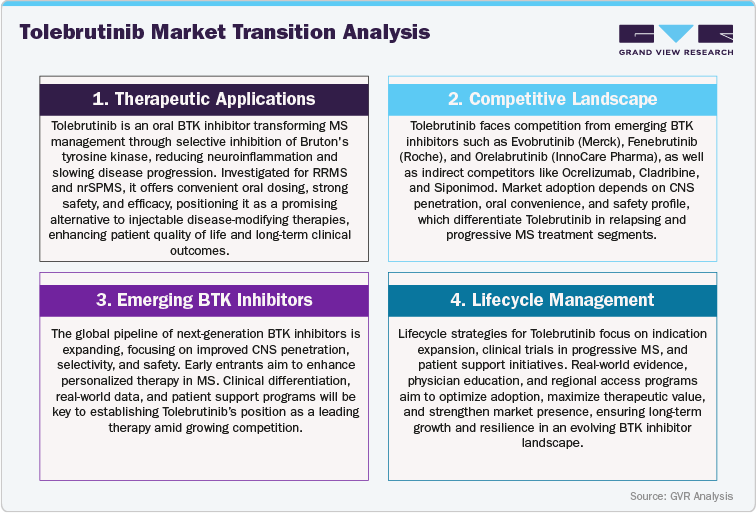
Tolebrutinib vs. Existing MS Therapies and Emerging BTK Inhibitors
Tolebrutinib and existing multiple sclerosis (MS) therapies differ significantly in mechanism of action, efficacy, and safety profile. Tolebrutinib, a selective Bruton's tyrosine kinase (BTK) inhibitor, modulates both B cell and myeloid cell activity, reducing neuroinflammation, demyelination, and subsequent axonal damage. Traditional disease-modifying therapies (DMTs), including injectable agents like interferons or monoclonal antibodies such as ocrelizumab and natalizumab, primarily reduce relapse rates but have limited impact on long-term disability progression. Clinical comparisons suggest that Tolebrutinib may offer a more favorable risk-benefit profile, with fewer systemic immunosuppressive effects and improved convenience due to oral administration.
Pharmacokinetically, Tolebrutinib provides distinct advantages over conventional MS therapies and emerging BTK inhibitors. Its oral, once-daily dosing enables consistent therapeutic exposure, simplifies adherence, and allows more flexible treatment optimization. In contrast, injectable therapies require frequent administration and monitoring, while some investigational BTK inhibitors may have longer half-lives or less selective immune modulation, increasing the potential for off-target effects. These differences highlight Tolebrutinib’s potential for safer, more patient-friendly, and individualized therapy.
Looking toward the future, several BTK inhibitors and other oral neuroimmunology therapies are in development, aiming to expand treatment options for relapsing and progressive MS. While promising, these emerging agents have not yet demonstrated the same degree of clinical efficacy, safety, or oral convenience as Tolebrutinib. Given its demonstrated benefits, including mechanism-based efficacy, tolerable safety profile, and pharmacokinetic flexibility, Tolebrutinib currently holds a competitive edge in the MS therapeutics landscape. Its profile positions it as a preferred option for clinicians seeking targeted, precision-based therapy for patients with both relapsing-remitting MS (RRMS) and non-relapsing secondary progressive MS (nrSPMS).

Opportunities and Strategic Challenges
The Tolebrutinib market presents significant opportunities driven by increasing demand for precision neuroimmunology therapies in multiple sclerosis (MS). The drug’s novel Bruton's tyrosine kinase (BTK) inhibitor mechanism allows differentiation from traditional disease-modifying therapies (DMTs), providing both relapse reduction and potential long-term neuroprotection. Early adoption by neurologists is supported by robust clinical data demonstrating efficacy, a favorable safety profile, and convenient oral administration. Market expansion is also influenced by rising awareness of MS, improved diagnostic rates, and the unmet need for targeted therapies for both relapsing-remitting MS (RRMS) and non-relapsing secondary progressive MS (nrSPMS). Sanofi’s strategic focus on patient education, adherence programs, and medical outreach further strengthens its competitive position. However, careful planning is required to optimize pricing and reimbursement strategies to ensure both patient accessibility and commercial sustainability.
Long-term safety remains a critical consideration for Tolebrutinib’s adoption. While clinical trials show favorable outcomes, ongoing post-marketing surveillance will be necessary to monitor potential adverse effects over extended periods. Regulatory authorities may require additional real-world evidence to support broader approval and labeling. Sanofi’s continued investment in R&D allows for refinement of dosing protocols, evaluation of combination therapies, and exploration of new patient populations. Strategic collaboration with healthcare providers and payers will be essential to balance affordability with commercial viability. Ensuring comprehensive long-term data and effective risk management plans will enhance physician confidence and patient adherence, driving sustainable growth for Tolebrutinib.
Differentiation strategies will be pivotal to maintain Tolebrutinib’s competitive edge in a landscape that includes existing DMTs and emerging BTK inhibitors. Sanofi’s focus on targeted therapy, oral dosing, and precision-based treatment aligns with evolving neuroimmunology trends. Potential combination approaches with complementary medications could further expand therapeutic utility and market penetration. Strategic pricing, global commercialization partnerships, and efficient supply chain management are additional factors influencing adoption and profitability. By addressing clinical, commercial, and regulatory challenges, Sanofi aims to position Tolebrutinib as a preferred therapy for both RRMS and nrSPMS, maximizing its impact on patient outcomes and long-term market success.
Market Forecast and Future Outlook (2025-2035)
The global Tolebrutinib market is poised for substantial growth over the next decade, driven by increasing adoption in multiple sclerosis (MS) management. With anticipated approval in major markets, including the U.S., Europe, and Asia-Pacific, the drug is expected to achieve rapid penetration among both relapsing-remitting MS (RRMS) and non-relapsing secondary progressive MS (nrSPMS) patient populations. Rising awareness, improved diagnostic capabilities, and a strong focus on precision neuroimmunology will further accelerate uptake. Sanofi’s strategic partnerships and global commercialization efforts are designed to facilitate efficient distribution and access, supporting sustainable market growth. Analysts view Tolebrutinib as a transformative therapy capable of redefining treatment paradigms, positioning it as a cornerstone in the evolving global MS therapeutics landscape.
Adoption trends indicate increasing utilization in both newly diagnosed and existing MS patients. Clinicians are expected to favor Tolebrutinib due to its oral administration, efficacy, and favorable safety profile. Expansion into nrSPMS represents a significant market opportunity, tapping into a previously underserved patient population with limited treatment options. Real-world evidence, ongoing post-marketing studies, and educational initiatives will reinforce confidence in treatment outcomes. The combination of targeted therapy, personalized dosing, and patient support programs will drive broader acceptance and long-term adherence. These factors collectively strengthen Tolebrutinib’s position as a preferred therapy, supporting both clinical adoption and commercial sustainability in the evolving MS treatment landscape.
Looking ahead, Tolebrutinib is projected to capture a significant share of the global MS market, reflecting its potential to address unmet clinical needs across relapsing and progressive forms of the disease. Market expansion will be supported by continued investment in R&D, potential combination therapies, and global regulatory approvals. Pricing strategies, reimbursement alignment, and strategic partnerships will be critical to maximizing penetration and accessibility. By offering a precision-based oral therapy, Tolebrutinib is positioned to establish a leadership role in MS management. Its long-term impact will be measured by improved patient outcomes, enhanced quality of life, and its contribution to shaping the future of neuroimmunology therapeutics worldwide.

Case Study (Recent Engagement): GLP-1 Receptor Agonist Market Opportunity Assessment
Project Objective
A leading global life sciences client approached us to assess the market potential and commercialization strategy for GLP-1 receptor agonist therapies across type 2 diabetes and obesity indications. The project aimed to support strategic planning for a novel, oral GLP-1 pipeline candidate, with a focus on launch timing, competitive positioning, and regional expansion.
GVR Solution
-
Conducted an epidemiology-based revenue forecast (2021-2036) using patient flow and analogue modeling approaches across North America, Europe, Asia Pacific, and the Middle East.
-
Delivered product-specific movement and market share analysis for:
-
Tolebrutinib- used as a reference analogue for uptake modeling
-
Orforglipron (pipeline)- projected using analogue-based scenarios from comparable oral GLP-1 launches
-
-
Benchmarked key players such as Eli Lilly and Novo Nordisk across financial performance, product pipeline, and global rollout strategies.
-
Assessed country-level pricing, regulatory, and reimbursement dynamics, supported by a custom launch timeline and uptake forecast for Orforglipron, modeled analogously to prior GLP-1 innovations.
-
Provided outputs (Excel, PPT, dashboard) and ongoing strategic support tailored to the client’s internal planning and commercialization team needs.

Impact for Client
-
Created market models for launch planning and portfolio prioritization.
-
Guided product strategy with pricing, uptake, and competitor insights.
-
Identified growth markets and shaped regulatory and launch plans.
Why this Matters
-
Build analogue-based forecasts for emerging therapies
-
Provide product insights for pipeline drugs with no historical sales
-
Offer strategic guidance on market entry, launch, and clinical-commercial integration
We bring the same level of analytical rigor, therapeutic market expertise, and consultative flexibility to your assessment of the pancreatic cancer microbubble-based therapy market.
Analyst Perspective
Tolebrutinib represents a transformative therapy in the evolving multiple sclerosis (MS) landscape, offering a mechanism-based, oral treatment that addresses both relapsing-remitting MS (RRMS) and non-relapsing secondary progressive MS (nrSPMS). Its Bruton's tyrosine kinase (BTK) inhibitor mechanism differentiates it from conventional disease-modifying therapies by targeting B cell and myeloid cell activity to reduce neuroinflammation and slow disease progression. The drug’s clinical profile, including favorable safety, efficacy, and oral convenience, positions it as a preferred option over traditional and emerging therapies.
Tolebrutinib’s ability to capture both established and underserved MS populations, particularly nrSPMS patients, is a key driver of adoption. Strategic commercialization plans, supported by Sanofi’s partnerships and educational initiatives, are expected to facilitate global rollout, especially in the U.S., Europe, and Asia-Pacific regions. Continued R&D and post-marketing data will reinforce long-term safety, efficacy, and real-world applicability.
Rising disease prevalence, increasing awareness, and the shift toward precision neuroimmunology therapies are expected to drive robust growth. The drug’s integration into evolving MS treatment algorithms, combined with flexible dosing and patient support programs, positions Tolebrutinib to set a new standard of care. It is anticipated to improve quality of life for patients while reshaping market dynamics and driving adoption across both relapsing and progressive MS segments.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


