Immunohematology Market: Sector Players Investing In Expanding Geographical Reach To Commercialize Untapped Potential By 2024
Rising prevalence of target diseases, such as bleeding disorders, sickle cell anemia, and cancer is contributing to the market growth. These patients require regular and recurrent blood transfusions, thus making a definitive market for immunohematology. The daily requirement of the same in the U.S. is 36,000 units;7,000 platelet units; and 10,000 units of plasma. Approximately 21 million components are transfused per year in the U.S. This opportunity can be commercialized by industry players.
Rising awareness about safety, need, and ease of donation has resulted in a surge of voluntary donors across the globe. Almost 92 million donations were recorded in 2015 across the world from all types of blood groups. Moreover, around 6.8 million donors were from the U.S. Type O is the most frequently required group, especially in hospitals. Considering only the U.S. population, 38% population is eligible to donate regularly. Out of this, 10% donate and nearly 13.6 million units are collected from them. This reflects the huge scope of blood collecting activities and the resultant expansion of immunohematology market.
The top reasons for blood requirements are automobile accidents, heart surgeries, organ transplants, bone marrow transplants, and treatment of burn victims. Urbanization of the emerging markets has resulted in road accidents to be the ninth leading cause of death. The total global expenditure related to road accidents is estimated to be USD 518 billion. An average road accident victim may require as much as 100 pints of blood components for survival.
Immunohematology market, by end-use, 2013-2024 (USD Million)
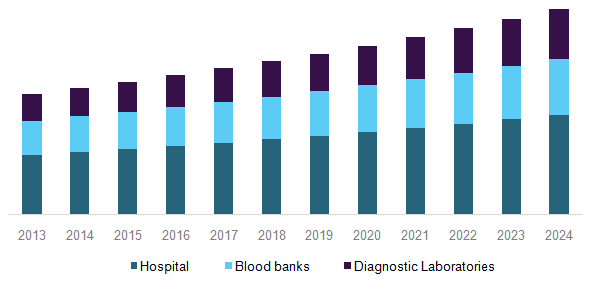
Blood transfusion is standardized by governments to minimize the risks of needle-acquired infections and complications due to excessive donations for patients and donors, respectively. As it cannot be manufactured, the only source that remains is the donor. This makes it crucial for end users to ensure the safety of the patients. It is scanned for type; viruses, such as hepatitis C & B, HIV, & parvovirus; and the count of its components. The compatibility between the donor and recipient also proves to be an important factor for a successful transfusion.
New product development is the key to cater to the growing demand. Industry players are in a constant process of innovation and launching new products to make the screening and characterization efficient and accurate. In March 2015, Roche launched next-generation viral load assay for HBV testing in Europe. The Cobas 6800/8800 System is a quantitative nucleic acid test with high sensitivity. The company also launched 14 diagnostic products out of which 5 were pertaining to hematology. Three were launched worldwide and few launched in the U.S. and European market.
 In-depth report on global immunohematology market by Grand View Research:
In-depth report on global immunohematology market by Grand View Research:
https://www.grandviewresearch.com/industry-analysis/immunohematology-market