Pulmonary Arterial Hypertension (PAH) Market - Increasing Patient Awareness About Disease & Diagnosis Will Drive Growth
Pulmonary hypertension is increase in blood pressure in the pulmonary arteries, veins and capillaries. As per the classification given by the Dana Point, WHO Group I represents PAH. There are many causes of PAH which may be idiopathic, drug and toxin associated, heritable, associated with diseases such as HIV infection, connective tissue disease, and portal hypertension. Group I is the largest subgroup, and there is no complete cure for this disease. Currently approved medications are largely limited improving on symptoms, quality of life, and slowing down the progression of disease.
The PAH is bifurcated in primary and secondary PAH. Primary PAH is the one which occurs without an identifiable cause. Secondary PAH occurs with some identifiable cause such as scleroderma, congenital heart diseases, HIV infection and liver diseases. The occurrence rate of primary PAH is 1-2 cases per million, while secondary PAH is observed in 10-50 cases per million.
Number of PAH patients is slowly increasing which can be attributed to the increasing awareness about disease and diagnosis.Players offering medication for PAH have increased significantly in past 10 years. Moreover, the diagnostic centers are upgraded with the latest available methods for PAH.
PAH Therapies
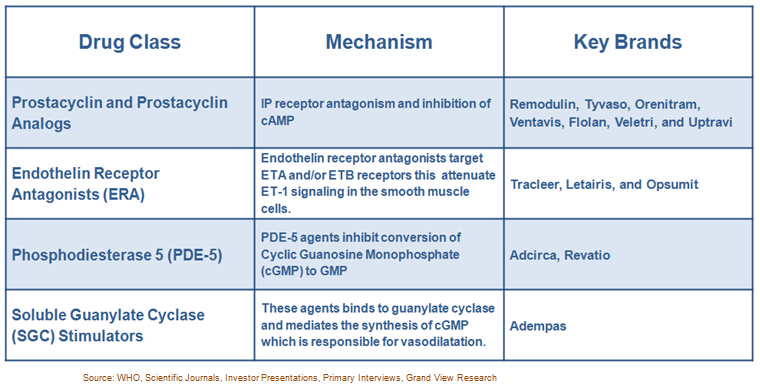
The U.S.FDA has approved 13 drugs to control PAH, these drugs can broadly be classified in four groups based on their mechanism of action. Prostacyclin and Prostacyclin Analogs, Soluble Guanylate Cyclase Stimulators, Endothelin Receptor Antagonists, Phosphodiesterase 5 are the classes for the treatment of PAH.
Following table summarizes the available therapies for treatment of PAH:
PAH Market: Key issue
Patent expiration of leading molecules is burning issue in PAH market, with low target population the market potential for these molecules is further reduced. The development process for a single molecule needs hefty investment of approximately around a few billion dollars and more than 10 years’ time. In addition, with stringent regulatory protocols and clinical scrutiny, it becomes more difficult. This is ultimately discouraging pharmaceutical companies to invest in R&D. Furthermore, in case of rare diseases, including PAH, the market size is relatively small and the drug developers are hesitant to invest time and money in the same. With the uncertainty about target population, it is difficult to recover all the investment and patent cliff adds on to this risk of profit recovery.
PAH Market: Reimbursement
The payment scrutiny for the orphan drugs including PAH agents is anticipated to increase as the new products are likely to hit the market over the forecast period. Moreover, due budget constraints in weakening economic scenario, the government is hesitant to cover rare diseases. The decision making is varied with factors such as cost, payer resources and benefit designs which ultimately have impact on patient access. Hence, clear understanding of economic and clinical value matrix in essential for decision making. It necessary for manufactures to develop strategies which ensure payers understand economic value of therapy thereby leading to accessibility by patients
 In-depth report on global pulmonary arterial hypertension pah market by Grand View Research:
In-depth report on global pulmonary arterial hypertension pah market by Grand View Research:
https://www.grandviewresearch.com/industry-analysis/pulmonary-arterial-hypertension-market