- Home
- »
- Clinical Diagnostics
- »
-
Antimicrobial Resistance Diagnostics Market Report, 2033GVR Report cover
![Antimicrobial Resistance Diagnostics Market Size, Share & Trends Report]()
Antimicrobial Resistance Diagnostics Market (2026 - 2033) Size, Share & Trends Analysis Report By Technology (Microbiology Culture, Immunoassay, PCR, NGS, Mass Spectrometry), By Pathogen, By End Use (Hospitals, Diagnostic Laboratories), By Region, And Segment Forecasts
- Report ID: GVR-4-68039-990-4
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2021 - 2024
- Forecast Period: 2026 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Antimicrobial Resistance Diagnostics Market Summary
The global antimicrobial resistance diagnostics market size was estimated at USD 3.42 billion in 2025 and is expected to reach USD 4.68 billion by 2033, growing at a CAGR of 4.08% from 2026 to 2033. The high prevalence of pathogens such as Streptococcus pneumoniae, MRSA, and C. difficile, along with the increasing number of antimicrobial-resistant (AMR) infections, is significantly driving market expansion.
Key Market Trends & Insights
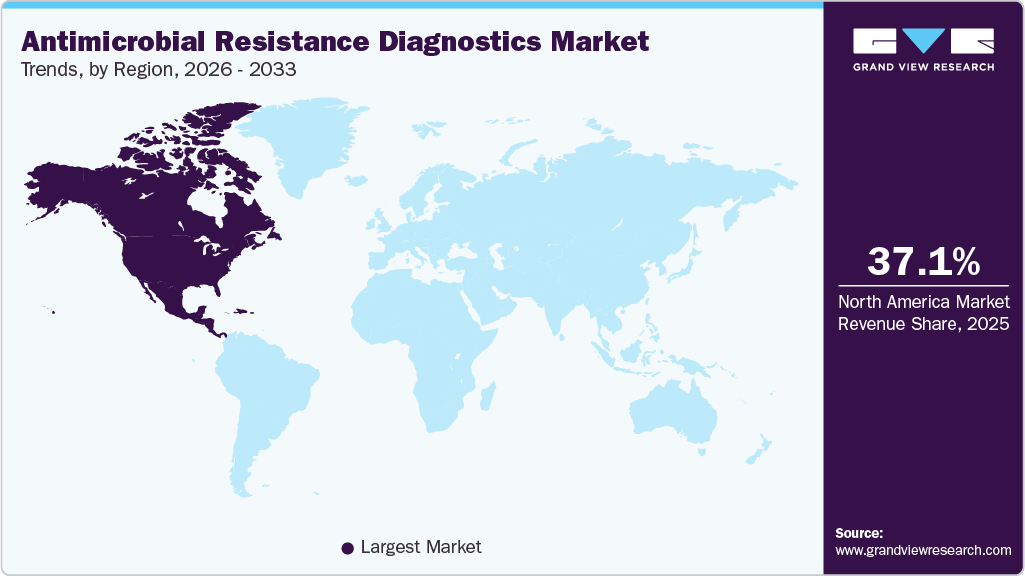
- The North America antimicrobial resistance diagnostics market accounted for the largest global revenue share of 37.06% in 2025.
- The U.S. antimicrobial resistance diagnostics industry led North America, with the largest revenue share in 2025.
- By pathogen, the Methicillin-Resistant Staphylococcus Aureus (MRSA) segment dominated the global market with the largest revenue share of 20.90% 2025.
- By technology, the microbiology culture segment held the largest revenue share of 51.68% in 2025.
- By end use, the hospitals segment held the largest revenue share of 58.20% in 2025.
Market Size & Forecast
- 2025 Market Size: USD 3,424.39 Million
- 2033 Projected Market Size: USD 4,680.97 Million
- CAGR (2026-2033): 4.08%
- North America: Largest Market in 2025
According to the CDC, approximately 2.8 million AMR cases occur annually in the U.S., and the U.S. Pharmacopeial Convention reports that AMR leads to around 700,000 deaths globally each year. This number could potentially rise to 10 million by 2050 if proper diagnosis and treatment of microbial infections are not implemented.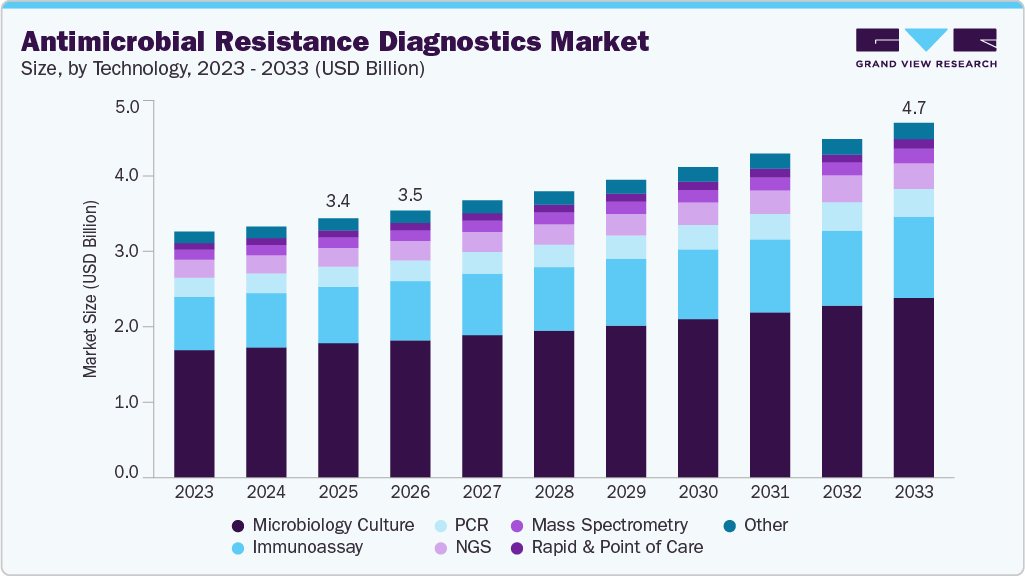
The rise in regulatory approvals for advanced and accurate diagnostic solutions is expected to meet the growing demand in the market. For example, in October 2021, Hologic, Inc. launched the fully automated Novodiag molecular diagnostic system from Mobidiag Oy in Europe. This system, which integrates microarray and real-time PCR technology, enables on-demand testing for antimicrobial resistance and infectious diseases, identifying multiple pathogens from a single sample. Additionally, in the same month, OpGen, Inc. received 510(k) clearance from the U.S. FDA to market its Acuitas AMR Gene Panel. This panel tests for 28 genetic antimicrobial resistance markers across various pathogens, aiding healthcare providers in managing resistant bacterial infections.
Recent surveillance-based prevalence of key drug-resistant pathogens
Pathogen
Metric (value)
Drug Resistant Streptococcus pneumoniae (DRSP)
Penicillin non-susceptibility 49.9% (non-susceptible = intermediate + resistant)
Drug Resistant Campylobacter (DRC)
Ciprofloxacin resistance among poultry isolates 68.8%
Clostridioides difficile (CD)
Hospital-based CDI incidence 6.2 cases per 10,000 patient-days
CPE (Carbapenemase-producing Enterobacterales)
Population-weighted mean carbapenem resistance in invasive K. pneumoniae 0.4% (EU/EEA mean)
VRE (vancomycin-resistant Enterococci)
Estimated prevalence 14.1% (period estimate from meta-analysis)
MRSA (Methicillin-resistant Staphylococcus aureus)
MRSA proportion among S. aureus isolates 26.39%
Drug-Resistant Neisseria gonorrhoeae (DRNG)
Ceftriaxone resistance 27% among 352 isolates
Drug-Resistant Salmonella (DRNTS)
Outbreak cases: 47 ceftriaxone-resistant S. Typhi cases
Others (aggregate signal)
One in six laboratory-confirmed bacterial infections resistant to at least one antibiotic
Moreover, leading players in the antimicrobial resistance diagnostics industry are collaborating to develop innovative solutions that address drug-resistant infections. For instance, in July 2022, Boehringer Ingelheim, bioMérieux, and Evotec SE formed a joint venture to develop actionable diagnostics and next-generation antibiotics to combat antimicrobial resistance. As part of this initiative, bioMérieux will focus on developing testing solutions for antibiotic resistance, with 80% of the investment dedicated to fighting AMR. Additionally, in June 2024, Sysmex Astrego was announced as a winner of the Longitude Prize for its PA-100 AST System, which is a point-of-care test for urinary tract infections.
Governments worldwide are working to enhance diagnostic quality for accurate and point-of-care diagnoses. For example, the UK government has launched initiatives to incentivize R&D for new AMR diagnostics. By 2024, the UK aims to bridge R&D gaps, collaborate with global stakeholders to develop next-generation diagnostic tests, and promote AMR diagnostics research. In February 2020, the UK invested approximately USD 12 million in partnership with Nigeria to combat drug-resistant infections by upgrading laboratory equipment, improving public health surveillance, and training scientists and technicians.
Furthermore, vendors have moved from promising proofs of concept to regulated, cleared products for rapid phenotypic AST and integrated ID plus AST workflows. A clear example is the June 21, 2024, US FDA 510(k) clearance of bioMérieux’s VITEK REVEAL system that reports actionable phenotypic susceptibility directly from positive blood cultures within a single work shift, facilitating same-day therapeutic adjustments. At the same time, Sysmex publicly documented compact AST systems in 2023 and 2024 that yield presence calls and antimicrobial efficacy results from urine samples in under 30 minutes, demonstrating how microfluidics can be applied to bedside decision-making. These concrete launches alter the procurement calculus by reducing validation risk for hospital labs.
Market Concentration & Characteristics
Innovation in AMR and DRNG diagnostics has accelerated in recent years, driven by the urgent need for rapid resistance detection and targeted therapy. On 18 March 2024, Visby Medical announced the launch of its single-use, patient-side PCR test for gonorrhoea with resistance-marker detection, advancing same-visit decision-making at the point of care. Earlier, SpeeDx expanded the market availability of its ResistancePlus GC assay in the EU, Australia, and New Zealand, enabling the molecular prediction of ciprofloxacin susceptibility by detecting gyrA S91F mutations, thereby supporting the reuse of oral ciprofloxacin in select patients.
Mergers and acquisitions in AMR and broader infectious disease diagnostics have primarily focused on companies seeking molecular capabilities, automation, and expansion of rapid test portfolios. In February 2024, bioMérieux completed the acquisition of LuminUltra Technologies to strengthen its position in rapid microbial detection and environmental surveillance, reinforcing its AMR-related capabilities. In January 2023, Thermo Fisher Scientific finalised its acquisition of The Binding Site, expanding its specialty diagnostics footprint, including immunodiagnostic tools relevant to infection management and antimicrobial stewardship.
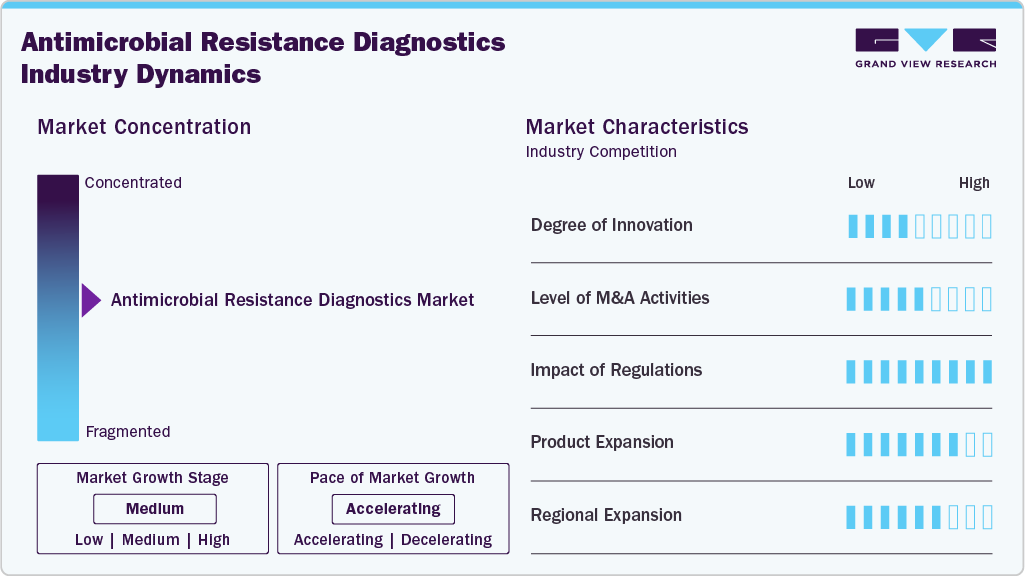
Regulation has played a defining role in shaping market entry and adoption of DRNG and AMR diagnostics. Under the European IVDR transition, assays predicting antimicrobial resistance, including those for DRNG, now require more extensive analytical and clinical validation, which slows the time to market but improves reliability. In the United States, the FDA’s shift away from pandemic-era Emergency Use Authorizations has tightened requirements for molecular assays, and point-of-care AMR diagnostics must meet full regulatory standards.
Diagnostic companies have expanded their AMR-related test menus, particularly in molecular assays capable of predicting resistance profiles. SpeeDx continues to expand the availability of its ResistancePlus GC test across multiple geographies, supporting clinical programs that allow selective use of ciprofloxacin. In June 2024, Seegene expanded its Allplex AMR assay portfolio, including enhanced panels for detecting fluoroquinolone resistance. In March 2024, Visby Medical introduced a palm-sized PCR test for gonorrhoea with resistance markers, representing a major shift toward ultra-portable, disposable molecular diagnostics.
Global demand for AMR diagnostics is driving geographic expansion by major players. SpeeDx achieved broader regulatory recognition for its ResistancePlus GC test across the EU, Australia, and New Zealand, allowing clinicians in these regions to access resistance-guided therapy. In 2023 and 2024, WHO and FIND-led initiatives encouraged the expansion of DRNG testing capacity in Africa and Southeast Asia, regions where ceftriaxone-resistant gonorrhoea outbreaks have been on the rise. Visby Medical’s recognition in the 2024 federal AMR diagnostic competition has accelerated interest from US healthcare systems preparing for point-of-care deployment once regulatory approval is achieved. These expansions signal a gradual global shift toward strengthening DRNG detection infrastructure, particularly in regions with high disease burden.
Technology Insights
The microbial culture segment dominated the antimicrobial resistance diagnostics market, accounting for the largest revenue share of 51.68% in 2025. The microbiology culture segment continues to drive the market growth as it remains the only universally accepted starting point for confirmed pathogen isolation and phenotypic antimicrobial susceptibility testing. Even with the rapid expansion of molecular and sequencing options, hospitals and reference laboratories still depend on culture to generate viable organisms for drug-susceptibility reporting, epidemiological assessment, and public health notification. This dependency means culture-based consumables, automation systems, and AST cards maintain consistent purchasing cycles across high- and middle-income markets.
However, the rapid and point-of-care (PoC) diagnostic segment is projected to experience the fastest compound annual growth rate (CAGR) of 5.47% during the forecast period. The increasing demand for PoC and rapid diagnostics is driven by their benefits, including quick turnaround times, fast results, and cost-effectiveness. The increasing demand for PoC and rapid diagnostics is driven by their benefits, including quick turnaround times, fast results, and cost-effectiveness. Recent product launches and innovations reinforce the shift toward immediate, bedside, or near-patient diagnostics for antimicrobial resistance and acute infections. In June 2023, Sysmex announced the launch of a point-of-care system in Europe capable of delivering antimicrobial susceptibility test (AST) results from urine samples in as little as 30 minutes, a significant reduction compared with conventional AST times that often take days.
Pathogen Insights
In 2025, the methicillin-resistant Staphylococcus aureus segment led the antimicrobial resistance diagnostics industry, accounting for the largest share of 20.90%. The rising incidence of MRSA in hospital settings is a key driver for the expansion of this segment. Hospitalized MRSA cases are projected to increase from 714,000 to 791,000 by 2033 across seven major markets, including the U.S., Japan, and European countries, driven by factors such as poor infection control, antibiotic resistance, and demographic changes. According to the CDC, approximately 33% of people carry Staphylococcus aureus in their noses without showing symptoms; however, about two out of every 100 of these individuals become infected with MRSA. Additionally, an increase in regulatory approvals for diagnostic tests targeting MRSA detection is further contributing to this growth.
The Drug-Resistant Neisseria Gonorrhoeae (DRNG) segment is likely to grow with a lucrative CAGR of 6.06% over the forecast period. Gonorrhoea remains one of the world’s most common bacterial sexually transmitted infections, so the diagnostics market must service large testing volumes as well as targeted AMR surveillance. The WHO’s global STI data estimate about 82.4 million new Neisseria gonorrhoeae infections among adults aged 15-49 in 2020. WHO’s surveillance programmes (GASP/EGASP) continue to document the emergence and spread of strains with decreased susceptibility to ceftriaxone and with high-level azithromycin resistance in sentinel sites; WHO published the Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) guidance on 24 January 2024 to strengthen sentinel AMR surveillance.
End Use Insights
In 2025, the hospitals segment led the antimicrobial resistance diagnostics market, accounting for 58.20% of the revenue share. Hospitals remain the primary demand engine for antimicrobial resistance diagnostics because they manage the highest-acuity infections where rapid identification and susceptibility data directly influence patient outcomes. Rising rates of bloodstream infections, surgical-site infections, ventilator-associated pneumonia, and other hospital-onset infections push hospitals to expand adoption of rapid molecular tests, automated culture systems, and stewardship-aligned diagnostic workflows.
Additionally, greater access to advanced diagnostic technologies, affordability, and improved healthcare coverage are contributing to the segment's expansion. Pathogens like MRSA, CDI, and DRSP can cause serious conditions such as bloodstream infections, sepsis, pneumonia, and surgical site infections, making the high occurrence of these infections in hospitals a key factor supporting market growth. Furthermore, a study conducted in April 2022 by BD, Pfizer, and Merck revealed that over 270 hospitals in the U.S. saw a rise in antimicrobial resistance during the COVID-19 pandemic, highlighting the ongoing challenge posed by AMR infections in healthcare settings.
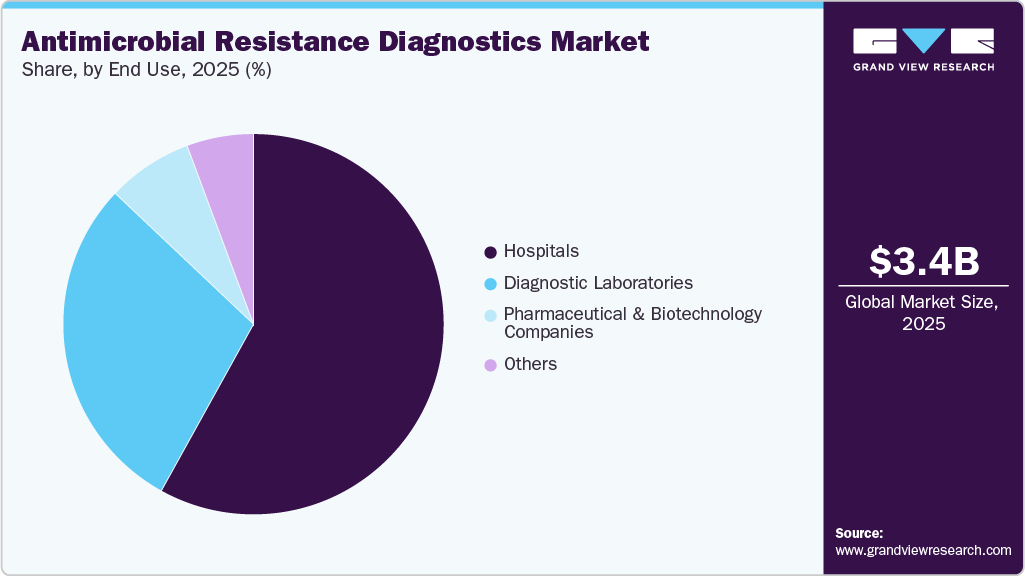
The diagnostic laboratories segment is expected to grow at the fastest CAGR of 4.93% over the forecast period. The growing prevalence of infectious diseases, combined with advancements in laboratory technology, is projected to propel segment growth. Additionally, the availability of affordable diagnostic services and the widespread presence of laboratories capable of conducting various diagnostic procedures are expected to contribute to market expansion. Laboratory testing plays a crucial role in patient management by identifying emerging threats and delivering accurate results essential for effective treatment. The CDC has also established its Antimicrobial Resistance (AMR) laboratory network to facilitate rapid resistance diagnosis and inform local authorities, helping to combat the spread of resistant infections.
Regional Insights
The North America antimicrobial resistance diagnostics market held the largest global revenue share of 37.06% in 2025. Growth in the region is supported by rising rates of drug-resistant infections, strong government initiatives, and well-established healthcare infrastructure. The presence of major diagnostic companies, along with ongoing product development and collaborations, further strengthens regional expansion. For example, in December 2024, the US Centers for Disease Control and Prevention expanded its AMR surveillance networks to include more laboratories and resistance pattern reporting, reinforcing the need for advanced diagnostics across hospitals and reference labs.
U.S. Antimicrobial Resistance Diagnostics Market Trends
The U.S. antimicrobial resistance diagnostics industry held a significant share of North America in 2025, fueled by the presence of key players and a large end user base. Furthermore, the U.S. invests heavily in research and development in the healthcare sector. In March 2025, the US National Institutes of Health announced additional funding for diagnostic innovation targeting priority AMR pathogens, supporting the development of new molecular and rapid testing solutions. These efforts are expected to strengthen market growth over the next several years. This investment fuels advancements in diagnostic technologies and the development of new solutions for combating antimicrobial resistance.
Europe Antimicrobial Resistance Diagnostics Market Trends
The Europe antimicrobial resistance diagnostics industry is experiencing significant growth. Many European countries face significant challenges related to antimicrobial resistance, with rising rates of drug-resistant infections. According to the UK Health Security Agency, nearly 400 new cases of antibiotic-resistant infections were reported per week in 2024, as published in November 2025 by the European Medical Journal. Government funding for innovation and AMR programs continues to rise, supporting the adoption of molecular and rapid testing platforms across the healthcare system. This situation drives the urgent need for effective diagnostic solutions to identify resistant pathogens and manage infections.
The UK antimicrobial resistance diagnostics market is experiencing significant growth due to advancements in healthcare and technological improvements. The rising incidence of drug-resistant infections has increased awareness among healthcare providers and policymakers about the urgent need for effective diagnostic solutions. The growing threat of drug-resistant infections in the UK is creating urgent demand for advanced diagnostic solutions to manage and contain antimicrobial resistance. For instance, according to an article published in the European Medical Journal in November 2025, new surveillance data from the UK Health Security Agency (UKHSA) indicate a significant increase in antibiotic-resistant infections, with nearly 400 new cases reported weekly in 2024.Furthermore, increasing government funding for healthcare innovation and antimicrobial resistance initiatives is driving the demand for antimicrobial resistance diagnostic solutions in the UK.
The Germany antimicrobial resistance diagnostics market is experiencing significant growth. Germany boasts a highly developed healthcare infrastructure, characterized by advanced laboratories and medical facilities, which facilitates the integration and adoption of innovative diagnostic technologies.
Asia Pacific Antimicrobial Resistance Diagnostics Market Trends
The Asia Pacific antimicrobial resistance diagnostics industry is experiencing the fastest growth, driven by significant advancements in healthcare infrastructure and technology. The increasing prevalence of infectious diseases and the rising incidence of drug-resistant infections in the region are the key factors for the market growth. Countries in Asia Pacific are grappling with significant public health challenges related to antimicrobial resistance, prompting a heightened need for effective diagnostic tools to identify resistant pathogens and manage infections effectively.
The China antimicrobial resistance diagnostics market is growing, driven by the rapid expansion of its biotechnology and pharmaceutical industries. China is making great strides in advancing its healthcare infrastructure, with significant investments in medical technology and diagnostic facilities. The rapid adoption of cutting-edge diagnostic tools, such as molecular testing and rapid point-of-care diagnostics, is helping healthcare professionals in China respond quickly and accurately to AMR challenges.
Latin America Antimicrobial Resistance Diagnostics Market Trends
The Latin America antimicrobial resistance diagnostics industry is experiencing significant growth, driven by increasing investments in the pharmaceutical and biotechnology sectors, particularly in countries such as Brazil and Argentina. Government initiatives aimed at combating antimicrobial resistance are also gaining momentum in the region. Several Latin American countries have developed national action plans that prioritize the surveillance of antimicrobial resistance, promote research and development in diagnostics, and encourage the appropriate use of antibiotics.
Middle East and Africa Antimicrobial Resistance Diagnostics Market Trends
The MEA antimicrobial resistance diagnostics industry is expected to grow over the forecast period. The regional growth is attributed to rising investments in the overall healthcare and life sciences sector, especially in countries such as South Africa, Saudi Arabia, and the UAE.
The Saudi Arabia antimicrobial resistance diagnostics market is expanding due to increased healthcare investment and improved infrastructure. Saudi Arabia's government has prioritized public health and is actively implementing strategies to combat AMR as part of its Vision 2030 initiative. This includes the establishment of national action plans to enhance surveillance, promote research, and improve the overall management of antimicrobial use.
Key Antimicrobial Resistance Diagnostics Company Insights
The competitive scenario in the antimicrobial resistance diagnostics market is high, with key players such as bioMerieux; F. Hoffmann-La Roche Ltd.; Abbott; Hologic, Inc.; BD; and Danaher holding significant positions. Prominent market participants are focusing on increasing the customer base using acquisition strategies. For instance, in April 2022, bioMérieux announced the acquisition of Specific Diagnostics. This acquisition has strengthened its position in the global market.
Key Antimicrobial Resistance Diagnostics Companies:
The following are the leading companies in the antimicrobial resistance diagnostics market. These companies collectively hold the largest market share and dictate industry trends.
- BD
- BIOMÉRIEUX
- Abbott
- Accelerate Diagnostics, Inc.
- Danaher
- Hologic Inc. (Gen Probe)
- Molsid
- F. Hoffmann-La Roche, Ltd.
- Vela Diagnostics
- Visby Medical, Inc.
- OpGen
- Seegene Inc
- EliTechGroup
- CERTEST BIOTEC
Recent Developments
-
In September 2025, Molecular Designs, a leading developer of molecular infectious disease assays, introduced the Urogenital Microbiota with ABX 53 Simplicity Panel, an advanced, multiplex PCR-based panel intended for research use that enables broad detection of urinary pathogens and antibiotic resistance (ABX) genes.
-
IN February 2025, CARB-X, the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator, announced a new, strategically focused funding call aimed at accelerating solutions for two critical unmet needs in global health. It focuses on advancing next-generation diagnostics for Salmonella Typhi, with the goal of enabling faster, more accurate detection to improve patient management and support public health efforts.
-
In November 2024, The Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) and the Clinton Health Access Initiative (CHAI) partnered to assess clinical requirements and overcome market challenges for diagnostic, preventive, and therapeutic solutions aimed at combating gonorrhea infections in low- and middle-income countries.
-
In October 2024, Seegene partnered with Werfen to establish a European hub, localizing syndromic real-time PCR product development and expanding molecular diagnostics in European markets.
Antimicrobial Resistance Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2026
USD 3.53 billion
Revenue forecast in 2033
USD 4.68 billion
Growth rate
CAGR of 4.08% from 2026 to 2033
Actual data
2021 - 2024
Forecast period
2026 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2026 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Technology, pathogen, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Norway; Denmark; Sweden; Turkey; Belgium; Netherlands; Switzerland; Portugal; China; Japan; India; Australia; Thailand; Vietnam; Malaysia; Indonesia; South Korea; Brazil; Argentina; Ecuador; Colombia; Peru; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
BD; bioMérieux; Abbott; Accelerate Diagnostics, Inc.; Danaher; Hologic Inc. (Gen-Probe); Molsid; F. Hoffmann-La Roche Ltd.; Vela Diagnostics; Visby Medical, Inc.; OpGen; Seegene Inc.; EliTechGroup; CerTest Biotec
Customization scope
Free report customization (equivalent up to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Antimicrobial Resistance Diagnostics Market Report Segmentation
This report forecasts revenue growth at the global, regional & country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the antimicrobial resistance diagnostics market report based on technology, pathogen, end use, and region:
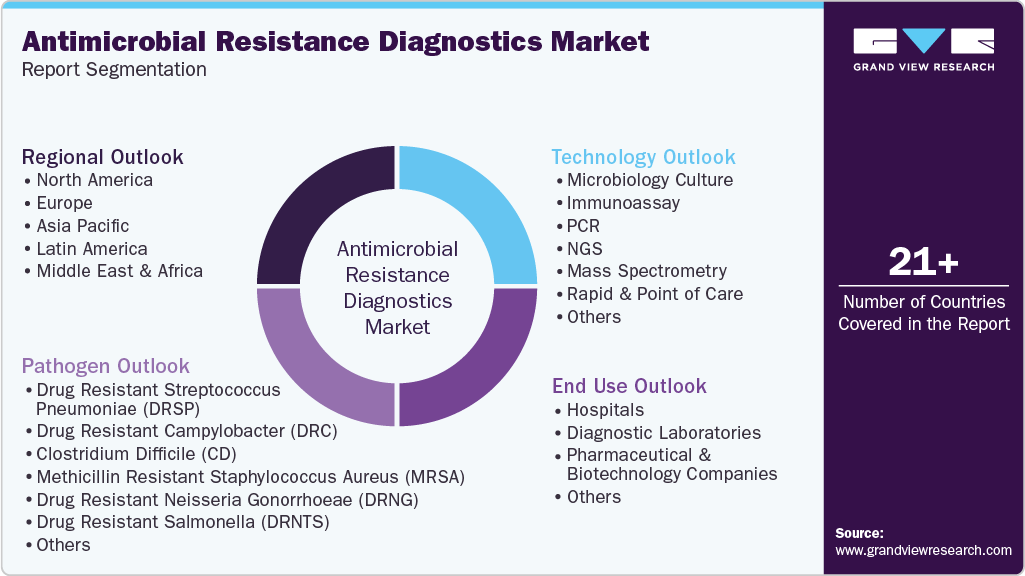
-
Technology Outlook (Revenue, USD Million; 2021 - 2033)
-
Microbiology Culture
-
Immunoassay
-
PCR
-
NGS
-
Mass Spectrometry
-
Rapid & Point of Care
-
Others
-
-
Pathogen Outlook (Revenue, USD Million; 2021 - 2033)
-
Drug Resistant Streptococcus Pneumoniae (DRSP)
-
Drug Resistant Campylobacter (DRC)
-
Clostridium Difficile (CD)
-
Methicillin Resistant Staphylococcus Aureus (MRSA)
-
Drug Resistant Neisseria Gonorrhoeae (DRNG)
-
Drug Resistant Salmonella (DRNTS)
-
Others
-
-
End Use Outlook (Revenue, USD Million; 2021 - 2033)
-
Hospitals
-
Diagnostic Laboratories
-
Pharmaceutical & Biotechnology Companies
-
Others
-
-
Regional Outlook (Revenue, USD Million; 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Norway
-
Denmark
-
Sweden
-
Norway
-
Portugal
-
Turkey
-
Switzerland
-
Belgium
-
Netherlands
-
Rest of Europe
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
Malaysia
-
Indonesia
-
Vietnam
-
Rest of Asia Pacific
-
-
Latin America
-
Brazil
-
Argentina
-
Colombia
-
Peru
-
Ecuador
-
Rest of Latin America
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global antimicrobial resistance diagnostics market size was estimated at USD 3.42 billion in 2025 and is expected to reach USD 3.53 billion in 2026.
b. The global antimicrobial resistance diagnostics market is expected to grow at a compound annual growth rate of 4.08% from 2026 to 2033 and is expected to reach USD 4.68 billion by 2033.
b. The microbial culture segment is expected to dominate the antimicrobial resistance diagnostics market with a share of 51.68% in 2025 as it remains the only universally accepted starting point for confirmed pathogen isolation and phenotypic antimicrobial susceptibility testing.
b. Some key players operating in the antimicrobial resistance diagnostics market include BIOMERIEUX, F. Hoffmann-La Roche Ltd, Abbott, BD, and Danaher among others.
b. The increasing risk of developing drug-resistant infections, the increasing introduction of technologically advanced tests, and government initiatives to reduce AMR disease burden are the major factors driving the antimicrobial resistance diagnostics market growth over the forecast period.
b. North America held the largest share of 37.06% in 2025 and is expected to register a lucrative growth rate over the forecast period. It is attributable to the supportive government policies to combat AMR, advanced healthcare infrastructure, and the presence of leading players in the region.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










