- Home
- »
- Medical Devices
- »
-
Bionic Eye Market Size, Share, Trends & Growth Report 2030GVR Report cover
![Bionic Eye Market Size, Share & Trends Report]()
Bionic Eye Market (2023 - 2030) Size, Share & Trends Analysis Report By Type (External, Implanted), By End-use (Hospitals, Ophthalmic Clinics), By Region, And Segment Forecasts
- Report ID: GVR-4-68038-849-7
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Bionic Eye Market Size & Trends
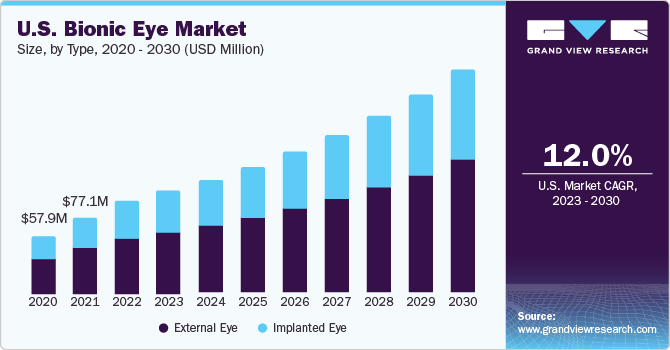
The global bionic eye market size was estimated to be USD 259.1 million in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 13.3% from 2023 to 2030. An increasing number of aging population, increasing prevalence of vision loss, product approvals, and intensive research and development focused on product innovation and advancement are expected to contribute to market growth. According to the World Health Organization, in October 2019, around 2.2 billion people were affected with vision impairment or blindness, of which at least 1 billion have unaddressed or preventable conditions worldwide. Positive outcomes of the clinical trials registered with bionic eye help accelerate the adoption of the product among people suffering from incurable eye diseases and permanent vision loss. In addition, positive results of the studies performed by conducting clinical trials on patients have proven safety, reliability, and long-term efficacy.
According to the National Eye Institute, retinitis pigmentosa (RP) is a rare genetic disorder that affects one in 4,000 people globally. According to Bright Focus Foundation, around 11 million people in the U.S. are suffering from some age-related macular degeneration, and the number is expected to double by 2050. The foundation further stated that the number of people with macular degeneration is estimated to reach 288.0 million by 2040, from 196.0 million in 2020 globally.
Increasing funding to help researchers develop novel ways to restore sight in blind individuals is a significant market growth contributor. In 2018, the Australian government granted funds from its BioMedTech Horizons program for commercializing a bionic eye developed by Monash Vision Group. The company commercialized its product by 2021, thus, addressing the unmet need for complete blindness.
Type Insights
Based on type, the market is categorized into external and implanted eyes segments. The external eye segment held the largest revenue share of over 60% in 2022, as it improves the visual experience. Technological advancements to provide better patient outcomes are a significant parameter focused by the market players. According to the National Institutes of Health, about three million Americans over the age of 40 are likely to suffer from macular degeneration by 2020.
Moreover, the external eye segment is anticipated to register the fastest CAGR of 13.6% over the forecast period, due to its better visual experiences than the other segments. Continuous advances in technology from various competitors in the industry, leading to cost reductions in the procedures, are expected to boost the market’s growth.
End-use Insights
Based on end-use, the market is segmented into hospitals, outpatient facilities, and research and manufacturing. The hospital segment held the largest revenue share of around 45% in 2022 and is anticipated to maintain its dominance over the forecast period. Hospitals offer the first line of care to the patients and are well-equipped with trained ophthalmologists and modern equipment to provide efficient patient care. Also, increasing medical coverage for the patient population and positive outcomes of surgeries are expected to increase the volume of surgical procedures, thereby augmenting the market growth.

The outpatient facilities segment is anticipated to register the fastest CAGR of 13.7%. This growth is anticipated due to the preference for outpatient facilities over hospitals. The collaboration between hospitals and product developers for conducting clinical trials has resulted in drastic growth of the segment.
Regional Insights
Based on region, the market is segmented into North America, Latin America, Europe, Asia Pacific, and Middle East & Africa. In 2022, North America held the largest market share of around 42%. The region is expected to continue with its dominance over the forecast period. North America has a favorable reimbursement scenario granting access to healthcare services, developed healthcare infrastructure, and intensive research activities. Thus, technology creates a significant impact on the market as it overcomes the challenge of printing electronics on curved surfaces. In 2018, researchers from Stanford University, in collaboration with Israel, received a grant from the U.S. National Institutes of Health and the U.S. Air Force. The primary focus was to develop a solar-powered retina to cure blindness caused by degenerative retinal disease- retinitis pigmentosa.
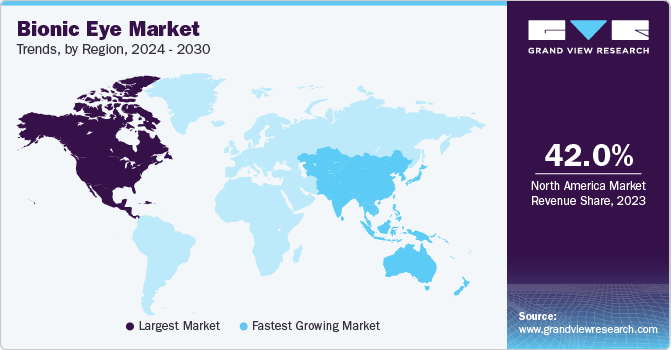
The market in the Asia Pacific region is anticipated to witness the fastest CAGR of 15.5% over the forecast period owing to the accelerated pace of the companies towards the launch of novel bionic eye implants. In January 2020, Bionic Vision Technologies, Australia announced pilot study results conducted in patients with late-stage retinitis pigmentosa for its bionic eye system- Gen3 device.
Key Bionic Eye Company Insights
Market players are adopting several strategies such as acquisitions to intensify the type of portfolio, geographical expansions, higher investment in research and development, and innovations to gain a significant share in the global market. For instance, in January 2022, Bionic Vision Technologies (BVT) announced the grant by the Food and Drug Administration (FDA) for its first bionic eye system in the U.S.
Key Bionic Eye Companies:
The following are the leading companies in the bionic eye market. These companies collectively hold the largest market share and dictate industry trends. Financials, strategy maps & products of these bionic eye companies are analyzed to map the supply network.
- Second Sight Medical Products LLC
- Nidek Co. Ltd.
- Nano Retina Ltd.
- MetaModal LLC
- Biomedical Technologies S.L.
- Bionic Vision Technologies
- Pixium Vision
- Monash Vision Group
Bionic Eye Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 291.6 million
Revenue forecast in 2030
USD 699.5 million
Growth rate
CAGR of 13.3% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Second Sight Medical Products LLC; Nidek Co. Ltd.; Nano Retina Ltd.; MetaModal LLC; Biomedical Technologies S.L.; Bionic Vision Technologies; Pixium Vision; Monash Vision Group
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Bionic Eye Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels as well as provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global bionic eye market report based on type, end-use, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
External Eye
-
Implanted Eye
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Outpatient Facilities
-
Research and Manufacturing
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global bionic eye market size was estimated at USD 259.1 million in 2022 and is expected to reach USD 291.6 million in 2023.
b. The global bionic eye market is expected to grow at a compound annual growth rate of 13.3% from 2023 to 2030 to reach USD 699.5 million by 2030.
b. North America dominated the bionic eye market with a share of 45.0% in 2022. This is attributable to rising healthcare awareness coupled with cloud-based technologies acceptance and constant research and development initiatives.
b. Some key players operating in the bionic eye market include Second Sight Medical Products LLC; Nidek Co. Ltd.; Nano Retina Ltd.; MetaModal LLC; Biomedical Technologies S.L.; Bionic Vision Technologies; Pixium Vision; Monash Vision Group.
b. Key factors that are driving the bionic eye market growth include increasing Medicare reimbursement for telehealth services, reducing emergency room visits and hospitalization rate, and technological innovation in communication technology across the world.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










