- Home
- »
- Medical Devices
- »
-
Cardiovascular Clinical Trials Market Size, Share Report 2030GVR Report cover
![Cardiovascular Clinical Trials Market Size, Share & Trends Report]()
Cardiovascular Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, Phase II, Phase III, Phase IV), By Study Design (Interventional, Observational, Expanded Access), By Indication, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-135-0
- Number of Report Pages: 195
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Cardiovascular Clinical Trials Market Trends
The global cardiovascular clinical trials market size was estimated at USD 5.26 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 6.0% from 2023 to 2030. The market's growth is majorly driven by the increasing prevalence of cardiovascular diseases such as heart failure, stroke, and coronary artery diseases. For instance, as per the World Heart Federation, cardiovascular diseases are one of the leading causes of mortality globally, resulting in around 20.5 million deaths in 2021. In addition, increased government and industry investments, coupled with a growing demand for cost reduction in phase III trials, are fueling market expansion.
The continuing innovations in medical research, such as personalized medicine and genomics, drive the demand for clinical trials to explore innovative approaches and treatments. The technological advancements in medical research, such as the adoption of telemedicine and wearable devices for virtual monitoring of clinical trial patients, are facilitating more cost-effective and efficient clinical trial management, thus supporting the growth of the market.
The outbreak of the COVID-19 pandemic had a negative impact on the market in 2020. The initial phase of the COVID-19 pandemic led to ongoing cardiovascular clinical trials being disrupted due to travel restrictions, lockdowns, and healthcare system strain caused by the pandemic. Moreover, the pandemic made recruiting and retaining participants in cardiovascular trials difficult. Limited access to healthcare facilities, fear of infection, and prioritization of COVID-19 patients posed difficulties to patient enrollment.
However, with an aim to rebound clinical trial revenues for cardiovascular treatment, several contract research organizations adopted virtual methods for clinical research, such as the incorporation of remote monitoring and telemedicine. This strategic initiative allowed several cardiology-based clinical trials to continue, with required adjustments in data collection methods and trial protocols.
The demand for improved cardiovascular treatments with new therapies and drugs is increasing the need for clinical trials on drugs that can help treat cardiovascular disease. Consequently, the high number of clinical trials with the latest treatments and therapies to treat the high burden of cardiovascular disease is elevating market growth. For instance, according to the Mayo Clinic, in 2023, the clinical trial for superficial cervical plexus block for pacemaker insertion is being carried out to investigate the clinical outcomes from two current standard-of-care pain control procedures performed for patients undergoing pacemaker insertion. Furthermore, in 2023, the World Health Organization mentioned that around 15 million individuals suffer from stroke, of which 5 million die, and the remaining 5 million become permanently disabled. The high burden of various cardiovascular diseases is promoting the need to conduct more cardiovascular clinical trials and provide timely treatment. The table below reflects the approximate number of cardiovascular-related deaths in the U.S.
2021
2022
Cardiovascular Related Deaths
695,000
699,659
Source: Centers for Disease Control Prevention and Grand View Research
Phase Insights
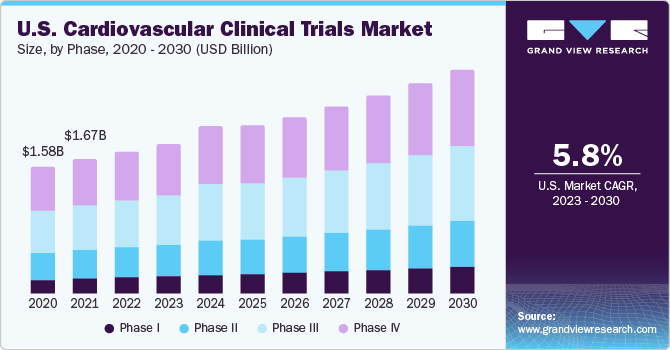
The phase IV segment dominated the market and accounted for the largest revenue share of 34.3% in 2022. Regulatory bodies such as the U.S. FDA and the European Union regulatory agencies may require post-marketing studies to ensure cardiovascular treatments' ongoing efficacy and safety. Compliance with these requirements drives the conduct of phase 4 trials. Moreover, a surge in patient data and genomic advances is increasing the demand for exploring the benefits of integrating real-world data into clinical trials. Also, as per experts across the industry, real-world data is most commonly integrated with phase IV clinical trials for payer approvals and health technology assessments. Hence, the aforementioned factors are anticipated to boost demand for phase IV trials, thereby augmenting segmental growth in the market.
The phase I segment is anticipated to witness a lucrative CAGR of 6.8% from 2023 to 2030. The growth is driven by increasing pipeline products about phase I clinical trials. The pharmaceutical pipeline has been strongly growing over the past few years, thus supporting the services for clinical trial development. The research and development (R&D) expenses are rapidly increasing to improve health care and provide new and improved cardiovascular treatments by conducting clinical trials. For instance, in June 2023, according to an article published by Pharma Manufacturing, the pharmaceutical industry's research and development (R&D) landscape is characterized by a significant drug pipeline, encompassing 21,292 drugs at various stages from preclinical development to post-market surveillance. Hence, high expenditure towards the pharmaceutical industry in elevating the growth of the cardiovascular clinical trial market, especially for phase I trials.
Study Design Insights
The interventional segment dominated the market and accounted for the largest revenue share of 64.8% in 2022. Interventional studies are categorized based on the intervention, including drug or biological, behavioral, surgical procedures, and devices. There has been a significant rise in interventional studies carried out over time. The interventional studies comprise 75.0 to 80.0% of total registered studies as of May 2022, most of which are drug or biologics, followed by behavioral, clinical procedure, and device intervention studies. Interventional studies are generally preferred for their greater accuracy and relevance than observational studies. Moreover, in terms of cardiovascular medical device clinical trials, interventional studies are the gold standard for the accuracy and efficacy of medical devices under study, thereby supporting the high segment shares in the market.
The observational segment is anticipated to witness a lucrative CAGR of 6.4% during the analysis period. Observational trials allow clinical trial specialists to gather real-world data on the impact of interventions or treatments performed in clinical practice beyond the controlled conditions of traditional clinical trials. These trials provide an enhanced perspective on treatment outcomes across several demographic groups by often involving more diverse and larger patient populations. Furthermore, observational trials are more cost-effective than interventional controlled trials, making them attractive for treatment studies that require large population statistics. In addition, these trials are considered a crucial element in post-market studies, as they help identify adverse events and safety concerns with the approved treatments. Hence, the aforementioned factors are anticipated to support the segment’s lucrative growth across the analysis time frame.
Indication Insights
The coronary artery disease category dominated the market and accounted for the largest revenue share of 20.0% in 2022. This is attributable to the growing number of coronary artery disease-related clinical trials that are under the process of evaluation. This is, in turn, proportional to the growing prevalence rate of the condition globally. For instance, the Centers for Disease Control and Prevention in 2023 stated that coronary heart disease is one of the most common types of heart disease in the U.S., and around 1 in 20 adults aged 20 and older have coronary artery disease. Hence, to meet the growing needs of the condition, several clinical trials are being conducted for the same, thereby supporting its robust revenue shares in 2022.
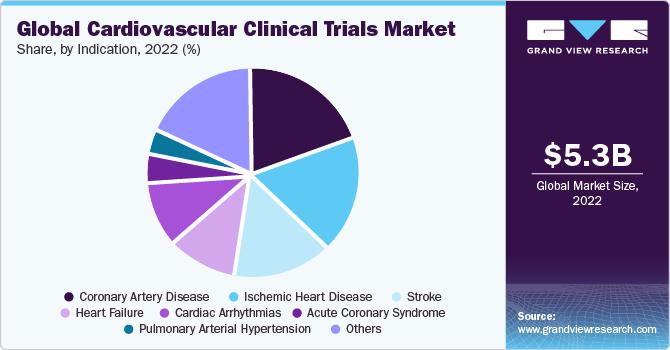
The stroke segment is anticipated to witness the fastest CAGR of 6.5% during the forecast timeframe. High growth is primarily due to the increasing rate of uncertain cardiac strokes being reported globally. It is important to note that developing economies such as India and China have witnessed a rigorous rate of cardiac stroke cases reported to the authorities in the past few years. For instance, in 2023, the Chinese Center for Disease Control and Prevention estimated that about 330 million patients suffer from cardiovascular diseases in China, with around 13 million patients suffering from a stroke. Hence, to overcome such medical needs, several types of research are being conducted across the countries and globally, thereby supporting the growth of the segment in the market.
Regional Insights
North America accounted for the largest share of 42.1% in 2022, owing to the presence of established CROs specializing in cardiovascular clinical trials, such as Worldwide Clinical Trials, ICON plc, Caidya, and several others. The U.S. is the biggest market for cardiovascular clinical trials. Several clinical trials for heart diseases are being conducted nationwide due to the presence of state-of-the-art infrastructure coupled with the adoption of several novel, ground-breaking technologies in clinical research. For instance, as per Clinicaltrials.gov, as of September 2023, around 5,068 ongoing cardiovascular clinical trials are registered in the U.S. Hence, the aforementioned factors are anticipated to support the lion’s share of the North America region.
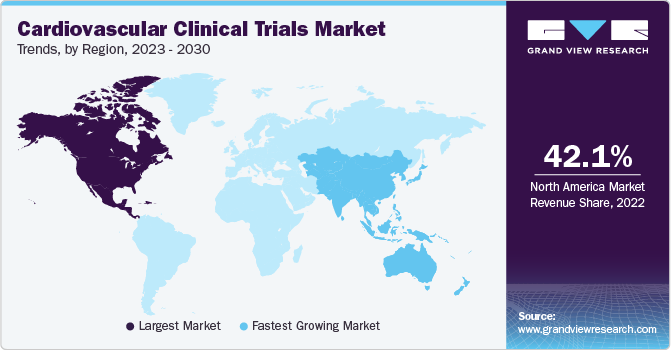
Asia Pacific is anticipated to register the fastest CAGR of 6.8% during the forecast period. Asia Pacific is one of the most attractive markets for conducting clinical trials due to their supportive regulatory reforms and cost-efficient alternatives for clinical research, especially in China and India. In addition, according to the Association of Standardized Patient Educators, the clinical research footprint is shifting to developing economies due to several factors, such as cost savings and shorter timelines, making it cheaper and easier to conduct trials outside the U.S. For instance, in 2022, according to the National Center for Biotechnology Information, India has become an increasingly popular country for international pharmaceutical companies to conduct clinical trials, as India has a higher ability to recruit patients and rapidly reduce clinical trial costs.
Key Companies & Market Share Insights
The major players operating across the market are focused on adopting in-organic strategic initiatives such as mergers, partnerships, acquisitions, etc. Moreover, companies focus on mergers and collaborations to better position themselves. For instance, in September 2023, Cereno Scientific, a biopharmaceutical company, announced a collaboration agreement with Clinical Trial Consultants (CTC), a contract research organization, to initiate a first-in-human Phase I study for CS014, a histone deacetylase inhibitor as a treatment for arterial and venous thrombosis prevention. Some prominent players in the global cardiovascular clinical trials market include:
-
IQVIA Inc
-
ICON plc
-
SGS SA
-
Eli Lilly and Company
-
PPD Inc
-
Syneos Health
-
Caidya
-
Worldwide Clinical Trials
-
Vial
-
Veeda Clinical Research
-
Medpace, Inc.
Cardiovascular Clinical Trials Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 5.58 billion
Revenue Forecast in 2030
USD 8.41 billion
Growth rate
CAGR of 6.0% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report Coverage
Revenue forecast, company share, competitive landscape, growth factors and trends
Segments Covered
Phase, study design, indication, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; India; Japan; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa, Saudi Arabia; UAE; Kuwait
Key companies profiled
IQVIA Inc; ICON plc; SGS SA; Eli Lilly and Company; PPD Inc; Syneos Health; Caidya; Worldwide Clinical Trials; Vial; Veeda Clinical Research; Medpace, Inc.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Cardiovascular Clinical Trials Market Report Segmentation
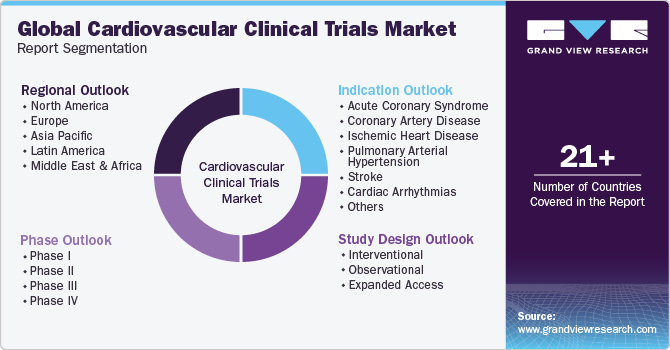
This report forecasts revenue growth at global, regional & country levels and provides an analysis on the industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global cardiovascular clinical trials market report based on basis of phase, study design, indication, and region:
-
Phase Outlook (Revenue, USD Million, 2018 - 2030)
-
Phase I
-
Phase II
-
Phase III
-
Phase IV
-
-
Study Design Outlook (Revenue, USD Million, 2018 - 2030)
-
Interventional
-
Observational
-
Expanded Access
-
-
Indication Outlook (Revenue, USD Million, 2018 - 2030)
-
Acute Coronary Syndrome
-
Coronary Artery Disease
-
Ischemic Heart Disease
-
Pulmonary Arterial Hypertension
-
Stroke
-
Cardiac Arrhythmias
-
Heart Failure
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global cardiovascular clinical trials market size was valued at USD 5.26 billion in 2022 and is expected to reach USD 5.58 billion in 2023.
b. The global cardiovascular clinical trials market is expected to grow at a compound annual growth rate of 6.0% from 2023 to 2030 to reach USD 8.41 billion by 2030.
b. North America dominated the market with a share of 42.1% in 2022. This is attributable to several pharmaceutical companies preferring to outsource their manufacturing services to CROs based in the U.S. This preference is driven by the consistent delivery of high-quality products and services by U.S.-based CROs.
b. Some of the few players include IQVIA Inc, ICON plc, SGS SA, Eli Lilly and Company, PPD Inc, Syneos Health, Caidya, Worldwide Clinical Trials, Vial, Veeda Clinical Research, Medpace, Inc. etc.
b. The cardiovascular clinical trials market's growth is majorly due to the increasing prevalence of cardiovascular diseases such as heart failure, stroke, and coronary artery diseases.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





